Pancreatoduodenectomy
CRITICAL ELEMENTS
Division of the Uncinate Process from the Superior Mesenteric Artery
Radical Lymphadenectomy
Resection and Reconstruction of the Superior Mesenteric Vein/Portal Vein to Obtain Negative Margins
Resection to Negative Margins at the Pancreatic Neck and Bile Duct
1. DIVISION OF THE UNCINATE PROCESS FROM THE SUPERIOR MESENTERIC ARTERY
Recommendation: The uncinate process of the pancreas should be dissected from the SMA along the periadventitial plane of the vessel. The right lateral aspect of the vessel should be skeletonized from the level of the first jejunal branch of the superior mesenteric vein (SMV) to the takeoff of the SMA from the aorta.
Type of Data: Primarily retrospective, low-level evidence.
Strength of Recommendation: Strong.
Rationale
The SMA courses from the aorta just to the left of the uncinate process as it enters the root of the small bowel mesentery. Thus, the vessel lies, at most, within millimeters of primary tumors of the proximal pancreas, and these tumors may infiltrate through the peripancreatic soft tissues into the perineural and lymphatic plexus surrounding the vessel. Resection and reconstruction of the SMA at the time of pancreatoduodenectomy is associated with unfavorable survival rates and is not recommended, even when it is performed in an attempt to clear the tissues around it of cancer and achieve a margin-negative resection.1 Furthermore, access to and dissection of the soft tissues
adjacent to the SMA are impeded by the venous confluence and pancreatic neck, both of which lie directly anterior to the artery. Therefore, meticulous dissection of the uncinate process from the SMA is simultaneously the most oncologically critical and technically challenging aspect of pancreatoduodenectomy.
adjacent to the SMA are impeded by the venous confluence and pancreatic neck, both of which lie directly anterior to the artery. Therefore, meticulous dissection of the uncinate process from the SMA is simultaneously the most oncologically critical and technically challenging aspect of pancreatoduodenectomy.
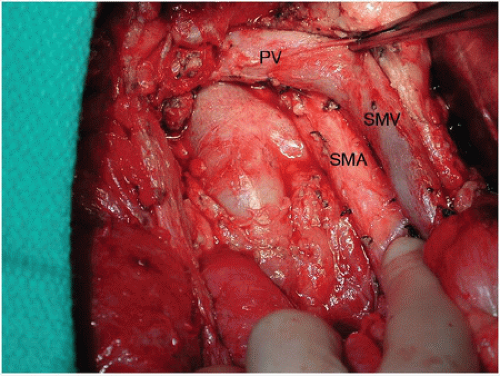
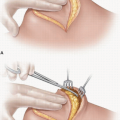
Tumor cells are found at the surgical margins in close to 90% of pancreatoduodenectomy specimens subjected to a rigorous pathologic protocol.2 The tissue between the uncinate process and SMA is the most likely anatomic location for a microscopically positive (R1) margin.3 Because meticulous dissection of the retropancreatic complex to the right of the SMA can minimize the amount of residual tissue on the artery, this technique may decrease, but not eliminate, the possibility of an R1 resection in well-staged patients (Fig. 14-1).4 The retropancreatic complex, which contains fatty tissue, lymphatics, and nerves—described as a “mesopancreas” by some3—can be resected in its entirety by skeletonizing the right lateral aspect of the SMA from the level
of the aorta to the level of the first jejunal branch of the SMV (Fig. 14-2). R0 resection rates achieved using this approach have not been directly compared with R0 rates achieved using a less radical dissection of the uncinate. However, no data suggests that this approach increases morbidity, and in fact the opposite may be true because the relevant vascular anatomy can be more fully appreciated using this meticulous technique.5 Routine dissection in this manner is therefore strongly recommended.
of the aorta to the level of the first jejunal branch of the SMV (Fig. 14-2). R0 resection rates achieved using this approach have not been directly compared with R0 rates achieved using a less radical dissection of the uncinate. However, no data suggests that this approach increases morbidity, and in fact the opposite may be true because the relevant vascular anatomy can be more fully appreciated using this meticulous technique.5 Routine dissection in this manner is therefore strongly recommended.
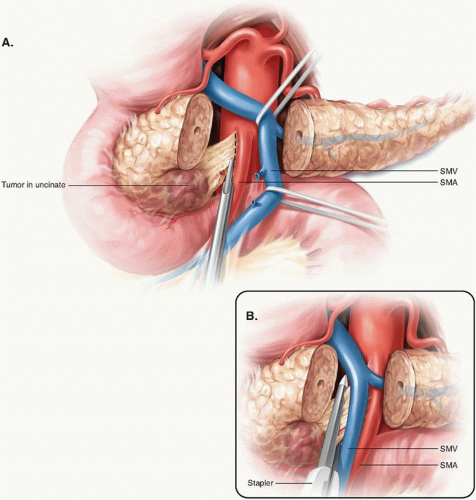
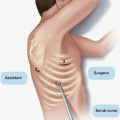
Some continue to advocate using a stapler to divide the uncinate from the SMA. In a small study in which 19 patients who underwent resection using a stapler were compared to 20 patients who underwent a more formal resection, neither group had a positive resection margin. However, the median number of lymph nodes retrieved and evaluated in each group (6.1 and 5.9 nodes, respectively) was low relative to that recommended by the American Joint Committee on Cancer (AJCC), suggesting that the histopathologic evaluation of the specimens had been suboptimal.6 Further, an anatomic study of cadavers revealed that the application of a surgical stapler to divide the uncinate from the SMA left up to 43% of the alveolar and lymphatic tissue on the SMA.5 Therefore, optimal dissection and skeletonization of the SMA requires the use of sharp, ultrasonic (harmonic scalpel), or thermal (LigaSure or Enseal) dissectors. The stapler must be avoided (Fig. 14-3).
Technical Aspects
Because the proximal SMA typically lies directly posterior to the SMV-portal vein (PV) confluence, the confluence must be completely mobilized to access the right lateral aspect of the SMA. For smaller tumors that minimally disturb the anatomy of this region, little dissection may be needed to retract the confluence to the left to expose the SMA. However, tumors that are in close proximity to either the SMV or PV often
require meticulous dissection to separate the vein from the pancreas. If the SMV or PV is inseparable from the head of the pancreas, vein resection may be necessary.
require meticulous dissection to separate the vein from the pancreas. If the SMV or PV is inseparable from the head of the pancreas, vein resection may be necessary.
If the SMV-PV confluence can be easily mobilized from the uncinate process, the specimen is gently retracted to the right, and the SMV-PV confluence is then swept to the left. This exposes the retropancreatic connective tissue. Meticulous dissection of the SMA proceeds either superiorly from the level of the first jejunal branch of the SMV or inferiorly from the takeoff of the SMA from the aorta (see Fig. 14-3). Although the caudal-to-cranial approach is more commonly described in the literature, a working knowledge of both techniques is necessary to safely deliver the specimen.
If the tumor is inseparable from the venous confluence, division of the pancreatic neck and leftward retraction of the confluence may be impossible. In this case, the pancreas may be divided to facilitate the exposure of the SMA; ligation of the splenic vein (SV) may also be performed to facilitate the rightward retraction of the confluence with the surgical specimen.7 Alternatively, dissection of the SMA may be performed using a posterior approach.8 No individual technical maneuver is mandatory; a combination of several maneuvers may be required for a safe, complete resection.
Once the SMA is completely exposed, all lymphatic, nervous, and adipose tissue lateral to the vessel is dissected to the right, and the inferior pancreaticoduodenal artery and branches are individually ligated. The relatively bloodless, periadventitial plane should be used for the dissection; circumferential dissection of the SMA is avoided to preserve the surrounding sympathetic plexus and minimize arterial injury. Skeletonization of the lateral, anterior, and posterior borders of the SMA to the level of the adventitia maximizes the yield of soft tissue adjacent to the SMA and presumably the likelihood of a margin-negative resection at this location.
CONCLUSION
The division of the uncinate process from the SMA is one of the most challenging operative components of pancreatoduodenectomy. A thorough working knowledge of the regional anatomy and its variations is key to performing a safe dissection. Complete mobilization of the SMV-PV confluence and dissection of the SMA margin along its periadventitial plane should maximize uncinate yield and thereby decrease the risk of an R1 resection.
2. RADICAL LYMPHADENECTOMY
Recommendation: A standard lymphadenectomy that includes the resection of nodes along the common bile duct, common hepatic artery, portal vein (PV), posterior and anterior pancreaticoduodenal arcades, superior mesenteric vein (SMV), and right lateral wall of the superior mesenteric artery (SMA) should be performed routinely at pancreatoduodenectomy.
Type of Data: Prospective, randomized (albeit flawed) studies, moderate-level evidence.
Strength of Recommendation: Strong.
Rationale
Pancreatic ductal adenocarcinoma (PDAC) has an extremely high propensity for both locoregional infiltration and systemic metastasis. The majority of tumors that appear localized on current state-of-the-art imaging are found to have regional lymph node involvement at the time of resection. Even among node-negative tumors, involvement of extrapancreatic lymphatic pathways within the retroperitoneal soft tissues is common.9,10
Lymphatic involvement is the most dominant pathologic prognostic factor for localized PDAC, and the quality of pathologic staging correlates strongly with the number of lymph nodes evaluated in the pathologic examination.11 Indeed, the primary
purpose of lymphadenectomy at the time of pancreatoduodenectomy is to obtain staging information. Current American Joint Committee on Cancer staging guidelines recommend that a minimum of 12 lymph nodes be analyzed, but others have argued that the examination of a minimum of 15 nodes is optimal for staging.12,13 Although higher lymph node counts have been correlated with better survival outcomes11 and one may postulate that lymphadenectomy impacts survival, the likelihood that extensive regional clearance significantly enhances survival is low given the propensity of PDAC toward early systemic dissemination.14 Nevertheless, it is sensible to perform a standard lymphadenectomy as part of pancreatoduodenectomy both to provide precise staging information and to optimize local control.
purpose of lymphadenectomy at the time of pancreatoduodenectomy is to obtain staging information. Current American Joint Committee on Cancer staging guidelines recommend that a minimum of 12 lymph nodes be analyzed, but others have argued that the examination of a minimum of 15 nodes is optimal for staging.12,13 Although higher lymph node counts have been correlated with better survival outcomes11 and one may postulate that lymphadenectomy impacts survival, the likelihood that extensive regional clearance significantly enhances survival is low given the propensity of PDAC toward early systemic dissemination.14 Nevertheless, it is sensible to perform a standard lymphadenectomy as part of pancreatoduodenectomy both to provide precise staging information and to optimize local control.
Standard Lymphadenectomy
Most peripancreatic lymph nodes are not visible intraoperatively, as they tend to be embedded within retroperitoneal adipose tissue. Thus, lymphadenectomy at the time of pancreatoduodenectomy conceptually is not a resective procedure guided by visible lymphatic anatomy; its extent is instead defined by vascular and visceral anatomic structures. The regional lymph node basins that are included in standard lymphadenectomy at the resection of tumors located in the pancreatic head and neck include nodes along the common bile duct, common hepatic artery, PV, posterior and anterior pancreaticoduodenal arcades, SMV, and right lateral wall of the SMA.13,15 The anatomy of the basins and their corresponding Japanese nomenclature are reported in Table 14-1 and depicted in Figure 14-4. The anatomic division of regional lymph nodes within the specimen is not necessary, but any nodes the surgeon separately submits for pathologic analysis should be reported as labeled by the surgeon.13 Completion of a standard lymphadenectomy should lead to acceptable lymph node counts (median, 13 to 17 nodes),16,17,18,19,20,21 provided that the surgical specimen is subjected to comprehensive pathologic analysis.
TABLE 14-1 Peripancreatic Lymph node stations | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||
Standard versus Extended Lymphadenectomy
Five randomized studies have now evaluated the possible benefits associated with extended lymphadenectomy over standard lymphadenectomy. These trials were performed in response to concerns that a more limited resection might leave residual cancer that could lead to local failure and poor survival, as well as in response to several prior retrospective studies that reported that a more extended lymphadenectomy offered a survival advantage over standard lymphatic dissection. These five studies’ designs differed to some degree, and the studies were inconsistent in terms of both the lymph node basins considered to comprise an extended lymphadenectomy and the technical methods used to perform the resections. Furthermore, each of the first four studies that were performed has been individually characterized as being underpowered. These limitations notwithstanding, none of the five studies showed that extended lymphadenectomy offered a clear benefit over standard lymphadenectomy in terms of long-term survival.16,17,18,19,20,21 Furthermore, associated quality of life analyses revealed that postoperative diarrhea occurred more frequently in patients who underwent extended lymphadenectomy than in patients who underwent standard lymphadenectomy. On the basis of these results, routine extended lymphadenectomy cannot be advocated.
Technical Aspects
During the Kocher maneuver used to mobilize the duodenum, the retroduodenal and retropancreatic soft tissues anterior to the vena cava are also mobilized. Although the routine resection of the aortocaval nodes between the level of the left renal vein and the lower duodenal border is not advocated, these nodes can be sampled and intraoperatively assessed if positive aortocaval lymphadenopathy is encountered, as survival following resection in this setting is particularly poor.22 Rather than simply transecting the lymphatic channels in this area, the surgeon should use clips, ligatures, or an energy device to seal these channels to prevent postoperative fluid buildup, which increases the risk for infection and chylous ascites.
After the gallbladder is mobilized en bloc to within the hepatoduodenal ligament, the pericholedochal tissues are resected below the level of the transection site of the common hepatic or bile duct; in addition, all soft tissues adjacent to the proper hepatic artery at this level and those anterior and lateral to the PV are removed en bloc. The removal of retroportal lymph nodes poses a special challenge because these nodes are frequently enlarged in the setting of biliary stenting or cholangitis; these structures posterior to the PV may best be left in place until the final steps of specimen mobilization.
Following the portal dissection, attention is turned to the dissection of the periduodenal and perigastric soft tissues. Depending on the plan for pylorus preservation, the specimen should include all tissue up to the site of gastric or duodenal transection and division of the right gastroepiploic or right gastric vessels. A lymph node is frequently encountered proximal to the origin of the gastroduodenal artery from the common hepatic artery, and this node marks well a transection plane within the soft tissue towards the pancreatic neck. Specific dissection of hepatic artery lymph nodes more proximal to this point is not routinely performed. In select cases, intraoperative sampling of the node may provide important prognostic information, as tumor involvement of this node is a poor prognostic factor for survival.23
After the pancreas neck is transected anterior to the PV and the distal duodenum and proximal jejunum are transected and mobilized, dissection continues along the SMV-PV confluence. All surrounding soft tissues are dissected, and small vascular branches are divided. This dissection exposes the soft tissues of the mesenteric root that surround the SMA. Unless the presence of tumor extension requires a different approach to be taken, the periarterial tissue is divided anterior to the artery course, and all soft tissues to the right lateral aspect of the SMA are mobilized with the specimen. The goal of this maneuver is to remove 180° of periarterial tissue to the level of the adventitia and to control small branches close to their origin. This facilitates the complete removal of the uncinate process parenchyma, the creation of the best possible margin clearance at the SMA, and the removal of all relevant lymph nodes in this area. This dissection should not be continued beyond the first jejunal branch of the SMA unless specific abnormalities of nodes within the mesenteric root are encountered. Similarly, transverse mesocolon nodes are not routinely resected unless specific findings suggest tumor involvement. The retropancreatic dissection is then completed from the SMA origin towards the liver hilus, and the retroportal lymph nodes can then be removed if necessary. Unless tumor encases it, any replaced right hepatic
artery originating from the SMA can usually be preserved even after the surrounding lymphoareolar and adipose tissues have been completely dissected. Final hemostatic maneuvers in all divided soft tissue areas complete the regional dissection.
artery originating from the SMA can usually be preserved even after the surrounding lymphoareolar and adipose tissues have been completely dissected. Final hemostatic maneuvers in all divided soft tissue areas complete the regional dissection.
3. RESECTION AND RECONSTRUCTION OF THE SUPERIOR MESENTERIC VEIN/PORTAL VEIN TO OBTAIN NEGATIVE MARGINS
Recommendation: The SMV, PV, or its confluence should be resected and reconstructed at pancreatoduodenectomy if the primary tumor is inseparable from the vessel and vascular involvement is all that impedes the performance of a margin-negative resection.
Type of Data: Primarily retrospective, low-level evidence and meta-analyses thereof.
Strength of Recommendation: Strong.
Rationale
During pancreatoduodenectomy for PDAC, vascular involvement of the tumor poses additional technical challenges to an already complex procedure. Preoperative planning for pancreatoduodenectomy must include high-quality pancreas protocol computed tomography with three-dimensional vascular reconstruction, which enables the surgeon to anticipate the need for venous resection and facilitates adequate preoperative preparation for vascular reconstruction. A review of the imaging should focus on assessing not only the relationship between the tumor and the surrounding major vessels, including the SMV and its first-order tributaries, but also the vascular anatomy itself, including evaluation for the presence of any anatomic variations, such as a replaced right hepatic artery.
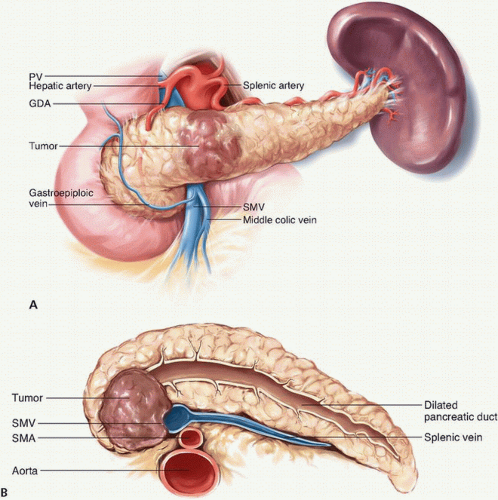
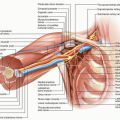
The PV courses posterior to the neck of the pancreas and arises from the confluence of the SMV and SV. The SMV originates from the confluence of the jejunal and ileal tributaries, which are adjacent to the uncinate process of the pancreas. Given the proximity of the vessels to tumors in the head, uncinate process, and neck of the pancreas, neoplastic involvement of these vessels is common (Fig. 14-5A,B). Attempts to skeletonize a primary tumor that is densely adherent to the vein may lead to vascular injury and/or macroscopically positive surgical margins (R2 resection). Although pancreatectomy with resection of the major mesenteric arteries carries five times the risk of perioperative mortality and twice the risk of death at 1 year compared with pancreatectomy without arterial resection, pancreatectomy with vein resection, when performed by surgeons experienced with the techniques required, results in morbidity and mortality equivalent to that of standard pancreatectomy.1,24,25,26 A recent meta-analysis of 19 previous studies estimated 1-, 3-, and 5-year overall survival rates of 61%, 19%, and 12%, respectively, for patients who underwent pancreatoduodenectomy with vein resection and 62%, 27%, and 17%, respectively, for patients who underwent standard pancreatoduodenectomy. This and other meta-analyses included heterogenous, nonrandomized studies; thus, the overall level of evidence is low. Regardless, pancreatoduodenectomy with vein resection and reconstruction should be
considered standard practice for nonmetastatic PDAC involving the SMV-PV confluence, provided that adequate inflow and outflow veins are present, the tumor does not involve the SMA or hepatic artery, and an R0/R1 resection can be reasonably expected.27 However, vein resection has no role as part of a “regional pancreatectomy,” initially described in the 1970s and 1980s, in which a greater amount of grossly uninvolved regional soft tissues is cleared in an attempt to improve outcomes.28,29
considered standard practice for nonmetastatic PDAC involving the SMV-PV confluence, provided that adequate inflow and outflow veins are present, the tumor does not involve the SMA or hepatic artery, and an R0/R1 resection can be reasonably expected.27 However, vein resection has no role as part of a “regional pancreatectomy,” initially described in the 1970s and 1980s, in which a greater amount of grossly uninvolved regional soft tissues is cleared in an attempt to improve outcomes.28,29
Intraoperative Margin Evaluation
Among patients who undergo venous resection for PDAC, patients with histologic tumor involvement of the resected vein have a poorer prognosis than patients without tumor involvement.30 However, the clinical significance of a positive venous transection margin (either tangential or segmental) is unclear. Given the logistical difficulties
associated with performing an intraoperative assessment of the vein margin while the portal system is cross-clamped, routine intraoperative analysis of the vein is therefore not recommended at present. The vein should be resected to grossly clear margins. Resecting clinically uninvolved vein is discouraged because doing so increases the likelihood that a simple lateral venorrhaphy or end-to-end vascular reconstruction must be converted to a more complex reconstruction involving a patch or interposition graft.
associated with performing an intraoperative assessment of the vein margin while the portal system is cross-clamped, routine intraoperative analysis of the vein is therefore not recommended at present. The vein should be resected to grossly clear margins. Resecting clinically uninvolved vein is discouraged because doing so increases the likelihood that a simple lateral venorrhaphy or end-to-end vascular reconstruction must be converted to a more complex reconstruction involving a patch or interposition graft.
Technical Aspects
In cases in which the pancreas can be transected at the neck, the extent of venous involvement may be appreciated as the head and uncinate process are separated from the SMV and PV by dividing the small venous tributaries from the pancreas. If the extent of tumor involvement is more pronounced, transection of the pancreas at the neck may not be possible; in such cases, the pancreas should be divided further to the left of the SMV (superficial to the SV) to prepare for segmental venous resection. The specimen is then separated to fully mobilize the SMV-PV confluence to identify the SMA over its entire proximal course. It may be advantageous to mobilize the specimen from the left side of the vein, separating it from the SMA following acquisition of adequate vascular control proximal and distal to the area of venous involvement. Regardless, it is best to defer final resection and reconstruction of SMV/PV until the entire specimen has been completely resected from all surrounding structures, including the SMA. This allows for better vascular control and minimizes the time needed for venous occlusion.
Options for SMV and/or PV resection and reconstruction depend on the site and extent of tumor involvement. If only a small portion of the lateral or posterior aspect of the SMV or PV is involved, the uninvolved uncinate process is separated from the vein, and a Satinsky clamp may be used to gain vascular control of the involved segment; after sharp transection of the involved vein, a primary repair with a 6-0 polypropylene suture can be performed if the lumen of the vessel is not compromised. If a primary repair would create significant narrowing of the venous lumen, a vein patch may be used to repair the SMV or PV (Fig. 14-6A).
If a short segment (≤2 cm) of the PV must be resected, a primary end-to-end anastomosis of the PV should be performed with preservation of the SV and inferior mesenteric vein (IMV) if possible (Fig. 14-6B). Additional PV length for a primary anastomosis can be gained by performing a complete Cattell-Braasch maneuver with mobilization of the retroperitoneal attachments of the small bowel and right colon mesentery up to the ligament of Treitz, which allows cephalad retraction of the root of the mesentery and the first-order SMV tributaries; division of the middle colic vein may also allow further mobilization of the SMV. In addition, easing the tension of a primary end-to-end PV anastomosis can be achieved by completely dissecting the falciform ligament and right triangular ligament and placing laparotomy pads above the liver, which allows for the caudal displacement of the proximal PV stump. Primary anastomosis of the SMV-PV confluence is usually not possible if the tumor involves the SMV below the level of the SV-PV confluence, as the SV prevents the caudal mobilization of the PV. Therefore, the resection of an SMV segment ≥2 cm with SV preservation generally requires an interposition graft (Fig. 14-6C). Autologous grafts created from the left internal jugular vein,31 superficial femoral vein,32 and left renal
vein33 have all been described. When the SV is preserved, access to the proximal 3 to 4 cm of the SMA is technically challenging, as the uncinate process attachments to the SMA in this region must be dissected posteriorly.
vein33 have all been described. When the SV is preserved, access to the proximal 3 to 4 cm of the SMA is technically challenging, as the uncinate process attachments to the SMA in this region must be dissected posteriorly.
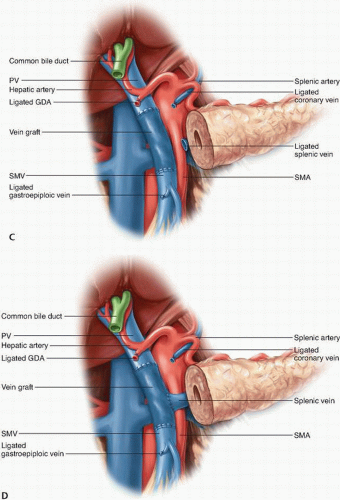
Management of the Splenic Vein. The SV may have to be divided to gain extra mobility for a direct end-to-end anastomosis between the PV and SMV or if tumor encasement is present at the level of the SV-PV confluence. If the IMV drains into the SV, the SV may be divided at its junction with the PV, as the IMV provides collateral venous drainage of the SV (Fig. 14-6D). However, if the IMV drains into the SMV, ligation of the SV can result in sinistral portal hypertension and increase the risk of future upper gastrointestinal hemorrhage. Various methods to reduce this risk have been reported, including reimplanting the SV into the side of the interposition vein graft (Fig. 14-6D),32 reimplanting the IMV into the SV,34,35 and performing an end-to-side splenorenal Warren shunt.36 Mandatory reconstruction of the SV remains a controversial practice, however, because studies suggest that portal hypertension is not inevitable after SV ligation without reconstruction.35 This has led many surgeons to adopt a selective approach for SV reconstruction.34,35
Management of the Jejunal and Ileal Tributaries of the Superior Mesenteric Vein
The SMV is formed by the confluence of the ileal and jejunal tributaries in more than 90% of individuals. The jejunal tributary, which drains the proximal small bowel, courses horizontally, typically posterior to the SMA, and enters the right posterolateral aspect of the main trunk of the SMV. The ileal tributary, which drains the distal small bowel, travels vertically through the small bowel mesentery. Anatomic variations in the first-order tributaries of the SMV and the drainage of the IMV are not uncommon, and the operating surgeon must be aware of these.
Isolated tumor involvement of the jejunal tributary requires the resection of this vein to allow for the mobilization of the uncinate process from its retroperitoneal attachments. Reconstruction of the resected jejunal tributary may not be necessary if the ileal tributary is of adequate diameter (i.e., around 1.5 times larger than that of the SMA as revealed by computed tomography37) to provide sufficient venous return to the small bowel. Tumor involvement of the SMV at the confluence of the jejunal and ileal tributaries generally requires resection and reconstruction of the veins. In this setting, the jejunal tributary is typically sacrificed because its thin walls and posterior, horizontal location make reconstructing it to the SMV difficult. Therefore, reconstruction between the ileal tributary and SMV is performed and often requires an interposition graft, especially if the SV has been spared.
Resection and Reconstruction
If possible, the specimen should be completely resected from all surrounding attachments, including the SMA, before resection and reconstruction of the SMV/PV. After the specimen has been removed, systemic anticoagulation is induced with 2,500 to 5,000 U of intravenous heparin. Complete vascular control is obtained by placing vascular clamps proximal and distal to the involved segment of vein and by clamping
the SV, IMV, and/or jejunal branches if necessary. Subsequent biliary and pancreatic reconstruction can be made less difficult by applying a Rummel tourniquet or soft bulldog clamp to the SMA to prevent inflow to reduce small bowel edema. The anterior walls of the SMV and PV and the interposition graft (when used) are inked proximally and distally with a marker to help orient the vein during reconstruction. The vein is transected, and the tumor is removed en bloc. The vein is then reconstructed using an interrupted or running 5-0 or 6-0 polypropylene suture with care taken to keep the inked walls aligned. Tying the running suture loosely is generally advocated, and when a graft is used, it is typically easier to complete the distal anastomosis first.
the SV, IMV, and/or jejunal branches if necessary. Subsequent biliary and pancreatic reconstruction can be made less difficult by applying a Rummel tourniquet or soft bulldog clamp to the SMA to prevent inflow to reduce small bowel edema. The anterior walls of the SMV and PV and the interposition graft (when used) are inked proximally and distally with a marker to help orient the vein during reconstruction. The vein is transected, and the tumor is removed en bloc. The vein is then reconstructed using an interrupted or running 5-0 or 6-0 polypropylene suture with care taken to keep the inked walls aligned. Tying the running suture loosely is generally advocated, and when a graft is used, it is typically easier to complete the distal anastomosis first.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree