Part B
Allergic Reactions to Individual Drugs: Low Molecular Weight
Allergic Reactions to Individual Drugs: Low Molecular Weight
Paul A. Greenberger
The approach described in this part of the chapter has been used successfully to permit treatment with both low- and high-molecular-weight drugs: β-lactam antibiotics among patients with a history of penicillin allergy and positive tests for the major and minor haptenic determinants of penicillin; among diabetic patients with systemic insulin allergy; and among patients with positive skin tests for heterologous antisera. Desensitization to these IgE-mediated reactions renders mast cells specifically unresponsive to only the drug antigen used for desensitization. In many patients, successful desensitization is accompanied by a marked decrease or transient disappearance of the cutaneous wheal-and-flare response. Similar changes in skin test responses have been reported following successful desensitization to vancomycin (1), aminoglycosides (2), and carboplatin (3).
The term desensitization has been used in its broadest sense to describe a state of unresponsiveness to a drug that is accomplished by repeated and increasing exposure to that agent (4,5). Similar to acute desensitization for IgE-mediated reactions, these patients have had undeniable reactions to these drugs in the past. In the absence of positive immediate skin test reactions converting to negative during the administration of the incriminated medication, the terms test dosing (4) or graded drug challenge (6) are recommended. For billing purposes, the only term is desensitization. Protocols have been described for the cautious administration of aspirin (7,8), sulfonamides (especially TMP-SMX and sulfasalazine) (9–12), allopurinol (13), tobramycin (14), and antiretroviral medications (10,15,16). Unlike desensitization to IgE-mediated reactions, these protocols are often more cumbersome and may require hours or days to complete (10). Finally, one should be reminded that graded dose challenges or true desensitization are potentially hazardous procedures best left to physicians experienced in managing hypersensitivity reactions (17). Highly trained nurses or other health care providers can participate in the monitoring of patients during challenges.
Test Dosing/Graded Dose Challenges
In situations in which a drug is needed and the history of a previous reaction to that agent is vague, and the possibility of true allergy is low, or the drug itself is an unlikely cause of such a reaction, test dosing or graded drug challenge is a method used to clarify the situation and safely determine whether it may be administered (17). A common example is a patient who has been advised to avoid all “caines,” and now requires the use of a local anesthetic agent. True systemic allergy to local anesthetics essentially is unheard of. Test dosing provides reassurance to the patient, physician, or dentist that this agent can be safely given. Alternatively, when the test dosing results in symptoms such as instantaneous throat closure without objective findings, it helps to confirm the level of anxiety involved without demonstrating true allergy.
The principle of test dosing (graded drug challenge) is to select a dose of the drug below that which would potentially cause a serious reaction, and then proceed with increasingly larger incremental doses to full therapeutic doses. Using this technique, one can determine whether a reaction has occurred before proceeding to the next dose. If a reaction occurs, it can be easily treated. If the drug is necessary, a desensitization protocol then may be performed. In this setting, controlled anaphylaxis can be carried out.
The starting dose, incremental increase, and interval between challenges depend on the drug and the urgency of reaching therapeutic doses (10). For oral drugs, a usual starting dose is 0.1 mg or 1.0 mg, and then proceeds to 10 mg, 50 mg, 100 mg, and 200 mg (10). For parenteral drugs, the initial dose is less, for example, 0.01 mg or 0.001 mg. When the suspected reaction was immediate, a 20- to 30-minute interval between doses is appropriate, and the procedure is usually completed in 3 to 5 hours or less. For late-onset reactions, such as a nonblistering or nonexfoliating dermatitis, the dosing interval may be as long as 24 to 48 hours, with the same protocols requiring 1 to 2 weeks or longer. Although there is always the possibility of a severe anaphylactic reaction, the risk of test dosing appears to be very low. Nevertheless, graded drug challenge of patients with a history of a bullous reaction to a medication or a serum sickness reaction (severe urticaria and arthralgia) (18) would have to be considered in rare patients, otherwise not attempted (10,17).
Special Considerations for Proven or Suspected Allergic Reactions to Individual Drugs
In this section, specific recommendations as they pertain to important drugs commonly used in clinical practice are reviewed. For each agent, relevant background information is provided. Table 17B.1 summarizes useful strategies for administering agents, once indication for the agent to be administered has been verified.
Table 17B.1 Examples of Useful Evaluation Techniques and Management Strategies for Selected Drugs and Agents | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Penicillins and Other β-Lactam Antibiotics
Background
β-Lactam antibiotic hypersensitivity deserves special consideration because of its medical importance. Penicillin has been studied extensively and has become a prototype for the study of allergic drug reactions. As many as 10% of hospitalized patients report a history of penicillin allergy. In a study of 1,893 consecutive adult patients who had an order written for an antimicrobial agent while hospitalized, 470 (25%) patients reported an allergy to at least one drug (19). Two hundred and ninety-five (15.6%) patients listed penicillin. A manual review of the charts revealed that just 32% of records specified the details of the allergic reaction. Some patients have been labeled falsely as penicillin allergic and are denied this useful, remarkably nontoxic agent. The reasons for this discrepancy are either a previously incorrect diagnosis or the frequently evanescent nature of penicillin allergy. Following an acute allergic reaction, there is a time-dependent decline in the rate of positive skin tests to penicillin. In the first year, 90% to 100% retain sensitivity after a convincing allergic reaction, but that percentage drops to about 30% at 10 years (20). It may be even lower in terms of cross-sectional studies of varying time periods. For example, penicillin allergy, confirmed by skin testing, can be 18% in penicillin allergic subjects (17,21). Some patients, however, maintain the penicillin-specific IgE antibody for 30 to 40 years. It is therefore highly desirable to predict which patients are at risk for a penicillin reaction. It is important to recognize that penicillin allergic patients, who have current penicillin sensitization by skin testing, may not have an “impressive” history of urticaria, angioedema, acute wheezing, etc. In a literature review, 347 of 1,063 (33%) patients, who tested positive on penicillin skin testing, had vague histories of penicillin allergy (22). These patients may well have had their level of risk from penicillin minimized.
The overall prevalence of β-lactam allergy is estimated to range from as high as 2% per course of treatment (23) to as low as 0.05% (24). The incidence of anaphylaxis has been reported to be as low as about 1:100,000 (24). The most frequent manifestations of penicillin allergy are cutaneous, notably morbilliform, and urticarial eruptions; the most serious is anaphylaxis. For historical comparison from the 1960s, penicillin-induced anaphylaxis occurred in about 0.01% to 0.05% (1 per 5,000 to 10,000) of patient treatment courses, with a fatal outcome in 0.0015% to 0.002% (1 death per 50,000 to 100,000 treatment courses) (25).
An atopic background (allergic rhinitis, asthma, atopic dermatitis) does not predispose an individual to the development of penicillin hypersensitivity, but once sensitized, such individuals are at increased risk for severe or fatal anaphylactic reactions (26). Anaphylaxis occurring in patients with asthma may result in acute severe respiratory failure. Also, atopic patients with Penicillium species “mold” allergy can receive penicillin unless specifically allergic to penicillin.
Patients with a history of prior penicillin reaction have a fourfold to sixfold increased risk for subsequent reactions to β-lactam antibiotics (27), but not necessarily as high to carbapenems, e.g., imipenem and meropenem (28,29). Among penicillin-allergic individuals who are skin test positive, the unmodified (regular infusion of the therapeutic dose) administration of β-lactam antibiotics causes acute reactions in about two-thirds of patients (30). If desensitization is performed, the incidence of reactions is very much lower (17,21). Graded
test challenge with imipenem and meropenem to patients with a history of penicillin allergy and positive immediate skin tests to penicillin or a determinant can be carried out safely when the skin tests to imipenem are nonreactive and the first dose of the antibiotic is 0.01 of the target dosage (28,29).
test challenge with imipenem and meropenem to patients with a history of penicillin allergy and positive immediate skin tests to penicillin or a determinant can be carried out safely when the skin tests to imipenem are nonreactive and the first dose of the antibiotic is 0.01 of the target dosage (28,29).
Although this discussion focuses primarily on the evaluation of and strategies to deal with IgE-mediated reactions, this group of agents has also been associated with other adverse, IgE-independent immunologic events that are briefly noted here and have been extensively reviewed elsewhere (11,16,17). Immediate reactions occur within the first hour following administration of the β-lactam drug, are IgE-mediated, and may present a serious threat to life. Accelerated reactions develop 1 to 72 hours after drug administration, are IgE-mediated, usually present as urticaria and angioedema, and are rarely life endangering. Delayed or late reactions occur after 3 days, are IgE-independent, and usually present as benign morbilliform skin eruptions. Exfoliative dermatitis and Stevens-Johnson syndrome may occur. Late reactions include serum sickness-like reactions (18) and drug fever (31,32). Unusual late reactions are immune cytopenias, acute interstitial nephritis, pulmonary infiltrates with eosinophilia, and hypersensitivity vasculitis.
In general, the previously described adverse events are common to all β-lactam antibiotics, such as the natural penicillins (penicillin G, penicillin V), the penicillinase-resistant penicillins (methicillin, nafcillin, dicloxacillin), the aminopenicillins (ampicillin, amoxicillin), and the extended spectrum penicillins (carbenicillin, ticarcillin, mezlocillin, azlocillin, piperacillin). Hypersensitivity reactions are less with the cephalosporins (10,11,17,33–35) and carbapenems (28,29). However, this statement is based on group mean statistics; the individual patient, who is destined to experience anaphylaxis from the antibiotic administration, is a “direct hit.” The data for carbapenems included graded challenges such as beginning with 1/100 then 1/10 of the target dose for imipenem (28).
Individual β-lactam antibiotics have been associated more commonly with certain types of reactions. For instance, ampicillin and amoxicillin therapy is associated with a higher incidence (about 10%) of nonpruritic maculopapular rash than are other penicillins (about 2%) (36). The rash usually appears after at least 1 week of therapy, initially develops on the knees and elbows, and then spreads symmetrically to cover the entire body (37). If the patient has infectious mononucleosis, the incidence approaches 90%. The incidence of this cutaneous reaction is increased in patients with HIV and cytomegalovirus (CMV) infection, chronic lymphatic leukemia, non-Hodgkin lymphoma, SLE, and hyperuricemia (38). This eruption does not appear to be allergic in nature, but if there is an urticarial component, it may represent true IgE-mediated penicillin allergy, and rechallenge could result in a severe immediate generalized allergic reaction.
Cephalosporins produce reactions similar to those described for penicillins. The more common reactions
include maculopapular or morbilliform skin eruption, drug fever, and a positive Coombs test (clinical hemolysis is unusual). Less common reactions are urticaria, serum sickness-like reactions (especially with cefaclor in children) (34,39–43), and anaphylaxis (34,44–48). Drug-induced cytopenias and acute interstitial nephritis are rare. Compared with the first-generation cephalosporins (e.g., cephalothin, cefazolin, cephalexin,* cefadroxil,* cefaclor*) and second-generation cephalosporins (e.g., cefamandole, cefuroxime, cefuroxime axetil*), the third-generation cephalosporins (e.g., cefotaxime, ceftizoxime, ceftriaxone, ceftazidime, cefixime*) have a lower incidence of immediate, presumably IgE-mediated, generalized allergic reactions (34).
include maculopapular or morbilliform skin eruption, drug fever, and a positive Coombs test (clinical hemolysis is unusual). Less common reactions are urticaria, serum sickness-like reactions (especially with cefaclor in children) (34,39–43), and anaphylaxis (34,44–48). Drug-induced cytopenias and acute interstitial nephritis are rare. Compared with the first-generation cephalosporins (e.g., cephalothin, cefazolin, cephalexin,* cefadroxil,* cefaclor*) and second-generation cephalosporins (e.g., cefamandole, cefuroxime, cefuroxime axetil*), the third-generation cephalosporins (e.g., cefotaxime, ceftizoxime, ceftriaxone, ceftazidime, cefixime*) have a lower incidence of immediate, presumably IgE-mediated, generalized allergic reactions (34).
* Oral agents.
Some degree of cross-reactivity (or independent sensitivity to non-β-lactam ring moieties) among the different classes of β-lactam antibiotics is well established (49). Because the semisynthetic penicillins contain the same 6-aminopenicillanic acid nucleus as natural penicillin G, it is not surprising that cross-allergenicity among these agents exists, albeit to various degrees. Individuals have been identified who have reacted to ampicillin and amoxicillin but not to penicillin (50–52). It is presumed that this is related to hypersensitivity to the side chains that differentiate the antibiotic from the parent compound. The incidence and clinical significance of these side-chain–specific reactions remains unknown. However, at this time, if a patient reports a history of penicillin allergy, it is prudent to assume that the individual is allergic to all penicillins (10,11,16,17). Because 9% to 25% of patients receiving antibiotics report a penicillin allergy (19,53–55), the impact of penicillin allergy in hospitalized patients remains significant.
Cephalosporins share a common β-lactam ring with penicillin but have a six-member dihydrothiazine ring instead of the five-membered thiazolidine ring of the penicillin molecule. Shortly after the introduction of the cephalosporins into clinical use, allergic reactions, including anaphylaxis, were reported, and the question of cross-reactivity between cephalosporins and penicillins was raised. Data suggest that the extent is not that high (56–59). Significant in vitro and in vivo cross-reactivity with penicillin has been demonstrated with first-generation cephalosporins (5% to 16.5%) (17,56–57). Fortunately, clinically relevant cross-reactivity between penicillin and the cephalosporins (especially second and third generation) is about 10% (17) and 2% to 3% (17), respectively. A literature review of patients with a history of penicillin allergy and positive skin tests to penicillin determinants challenged with cephalosporins revealed allergic reactions in 8.1% of patients compared with 1.9% among those with negative penicillin skin tests (59). A provocative review suggested that penicillin-allergic patients, who are identified by either history or positive penicillin skin tests, are not at increased risk compared with the general population, and they may be safely treated with cephalosporin antibiotics (35). However, cautious administration of cephalosporins to penicillin-allergic patients is advisable, especially when the history is that of acute urticaria or other anaphylactic reaction. Regrettably, in a report of six penicillin-allergic patients, three experienced fatal anaphylactic reactions from the first dose of a cephalosporin (47).
Primary cephalosporin allergy, including anaphylaxis, has occasionally been reported in both penicillin-allergic and penicillin-nonallergic patients and may be fatal (47). Most investigators have studied tolerance to the cephalosporins in penicillin-allergic patients, but little information is available regarding tolerance to other β-lactam antibiotics in patients with primary cephalosporin allergy. Such studies are limited by the lack of reliable cephalosporin determinants for skin testing. It appears that antibodies directed against unique side chains rather than against the common ring structure are more important in the immune response to cephalosporins (34,56–58). This would explain the low cross-reactivity among different cephalosporins, which share the same nucleus but have different side chains (34,56–59). Also, it may help to explain the low cross-reactivity between cephalosporins and penicillins, which share the same β-lactam ring in the nucleus but have different side chains. Until better information is available, it is best to avoid the use of β-lactam antibiotics in cephalosporin-allergic patients; if essential, cautious graded drug challenge is advisable. Although skin testing with the parent cephalosporin has not been used widely, initial reports of skin testing with the parent cephalosporin (2 mg/mL prick test, then 0.02 mL, intradermal) describe high negative predictive value (60).
The carbapenems (imipenem, meropenem), monobactams (aztreonam), and carbacephems (loracarbef) are three classes of antibiotics that possess β-lactam ring structures. There is significant cross-reactivity between penicillin and imipenem (61) and meropenem based on structure and using the specific determinants of impenem for example (61). Graded challenges were not carried out, but 47% of penicillin-allergic subjects had positive skin test reactions to imipenem determinants (61). Immediate skin reactivity to imipenem (1 mg/mL) by prick test was demonstrated in a patient who experienced shock and cardiac arrest from imipenem (62). Unexpectedly, there is much less actual, clinical cross-reactivity than anticipated between penicillin and the carbapenems, when the carbapenems are administered by graded challenge (28,29). Aztreonam is the prototypical monobactam antibiotic. It is very weakly cross-reactive in the penicillin-allergic patient and may be administered safely to most patients allergic to other β-lactam antibiotics (17,63). The antibodies generated are specific to the side chain rather than the β-lactam ring. It should be noted, however, that
ceftazidime, a third-generation cephalosporin, shares an identical side chain with aztreonam. It may be prudent not to use ceftazidime in rare subjects allergic to aztreonam. Loracarbef, a carbacephem, structurally resembles cefaclor, but the degree of cross-reactivity with penicillins and cephalosporins is unknown. Finally, clavulanic acid is also a β-lactam antibiotic with weak antibacterial activity but is a potent inhibitor of β-lactamase. It is often combined with amoxicillin to enhance antimicrobial activity. There is a report of two immediate generalized allergic reactions attributed to clavulanic acid (64).
ceftazidime, a third-generation cephalosporin, shares an identical side chain with aztreonam. It may be prudent not to use ceftazidime in rare subjects allergic to aztreonam. Loracarbef, a carbacephem, structurally resembles cefaclor, but the degree of cross-reactivity with penicillins and cephalosporins is unknown. Finally, clavulanic acid is also a β-lactam antibiotic with weak antibacterial activity but is a potent inhibitor of β-lactamase. It is often combined with amoxicillin to enhance antimicrobial activity. There is a report of two immediate generalized allergic reactions attributed to clavulanic acid (64).
Diagnostic Testing
Although obtaining and recording a past history of penicillin allergy is essential, one cannot completely rely on that information to predict who is allergic. The history may be inaccurate, and many patients lose their sensitivity over time. The failure to elicit this information has resulted in fatalities following the administration of these drugs to patients with a convincing history of β-lactam hypersensitivity. To help clarify this situation, when the drug is essential, skin testing with penicillin has been useful to identify those patients at risk for anaphylaxis and other, milder IgE-mediated reactions. When appropriate skin testing reagents are either unavailable or have not been validated, test dosing (graded drug challenge) with the desired β-lactam antibiotic is recommended.
Benzylpenicillin (BP) has a molecular weight of 300 and transforms (is not metabolized) in large part (about 95%) into a penicilloyl hapten moiety. This transformation product is referred to as the major determinant and has been conjugated to poly-D-lysine to form penicilloyl-polylysine (PPL), which had been commercially available as Pre-Pen (Hollister-Stier, Spokane, WA) for skin testing until 2004. Other penicillin transformation products, including BP itself, constitute 5% or less of administered penicillin and are collectively referred to as the minor determinant mixture (MDM). They are minor in name only but are responsible for some penicillin anaphylactic reactions. A standardized MDM is not available commercially for skin testing. Therefore, a fresh solution of BP (10,000 units/mL) has been used for skin testing purposes. Skin testing with both PPL and freshly prepared BP (as the sole minor determinant) should detect 85% to 88% of potential reactors (10,11,16,17,21). Almost all patients (99%) with negative skin tests to PPL and MDM reagents can be treated safely with penicillin (17,21). If PPL is not used but MDM is, from 34% to 60% of skin test-positive patients would be missed (17). Thus, the major determinant identifies a significant proportion of skin test–positive patients, and its use improves safety during testing and desensitization. With the PPL and MDM, the negative predictive value of skin testing with major and minor determinants is as high as 99% (17,21) compared with about 40% to 66% with MDM only (17).
In general, skin testing with BP-derived reagents, PPL and MDM, is also predictive of reactions to other β-lactam antibiotics (17); however, there are occasional patients with reactions to ampicillin, amoxicillin, and cephalosporin side chains who may not be detected by skin testing (50–52). Although skin testing with the β-lactam antibiotic of therapeutic choice has been advocated to detect additional potential reactors, skin test reagents prepared from other penicillins, cephalosporins, imipenem, and aztreonam have not been standardized, and the results are not validated. A positive skin test using these materials suggests the potential for an IgE-mediated reaction, but a negative test does not eliminate this concern. The incidence of such reactions to other β-lactam antibiotics when skin tests are negative to penicillin major and minor determinant reagents is probably low (17). Some minor determinant mixtures are not as sensitive as others and have led to confusion about the need to detect side-chain–specific IgE.
In practice, penicillin skin testing to evaluate the potential of or current risk for an IgE-mediated reaction should be reserved for patients with a history suggesting penicillin allergy when administration of the drug is essential or when confusion about penicillin interferes with optimal antibiotic selection. Such testing is of no value in predicting the occurrence of non–IgE-mediated reactions and is contraindicated when the previous reaction was Stevens-Johnson syndrome, toxic epidermal necrolysis (TEN), or exfoliative dermatitis. Elective penicillin skin testing followed by an oral challenge and subsequent 10-day course of treatment with penicillin or amoxicillin in skin test–negative subjects has been recommended, particularly in children with a history suggesting penicillin allergy (65). It was hoped that this procedure would eliminate the need to carry out such testing when the child is ill and in need of penicillin therapy. Using this approach, the risk for resensitization was about 1%. In one small study of 19 patients, 16% of penicillin history–positive, but skin test–negative adults receiving intravenous penicillin therapy became skin test positive 1 to 12 months after completion of treatment (66). In another study, none of 33 penicillin history–positive, skin test–negative adults had evidence of IgE-mediated reactions, suggesting loss of antipenicillin IgE antibodies (67). In a series of 568 patients with penicillin allergy and negative skin tests, only 1 of 33 patients, who were tested after the initial therapeutic course that resulted in a reaction, was skin test positive (68). These data suggest that reactions are not always IgE-mediated and that resensitization appears to be very low. The overall data support the use of penicillin skin tests in managing patients with a history of penicillin allergy, regardless of the severity of the previous reaction. Penicillin skin testing is rapid, and the risk for a serious reaction is minimal when
performed by trained personnel, using recommended drug concentrations, and completing skin-prick tests before attempting intradermal skin tests. Testing should be completed shortly before administration of the drug. However, in the absence of commercially available penicillin skin test reagents, the only option is to identify patients at higher risk than the normal population and perform graded drug challenges with caution.
performed by trained personnel, using recommended drug concentrations, and completing skin-prick tests before attempting intradermal skin tests. Testing should be completed shortly before administration of the drug. However, in the absence of commercially available penicillin skin test reagents, the only option is to identify patients at higher risk than the normal population and perform graded drug challenges with caution.
Table 17B.2 summarizes the reagents used for β-lactam antibiotic skin tests and the recommended starting concentrations of these reagents, which are adequately sensitive but have a low risk for provoking a systemic or nonspecific irritant reaction. In patients with a history of a life-threatening reaction to penicillin, it may be advisable to dilute the skin test reagents 100-fold for initial testing. Skin-prick testing is accomplished by pricking through a drop of the reagent placed on the volar surface of the forearm and observing for 15 to 20 minutes. A significant reaction is a wheal 4 mm or larger than the control with surrounding erythema. If negative, proceed with intradermal skin tests. Using a tuberculin or allergy syringe, inject 0.01 mL to 0.02 mL of the reagent, sufficient to raise a 2-mm to 3-mm bleb on the volar surface of the forearm. After 15 to 20 minutes, a positive test produces a wheal of 4 mm or larger with surrounding erythema. If the results are equivocal or difficult to interpret, the tests should be repeated. It should be noted that there is some disagreement among investigators as to what constitutes an acceptable positive skin test (20). A 4-mm wheal with surrounding erythema is positive; a 4-mm or greater wheal without erythema is “indeterminate” and usually not representative of antipenicillin IgE antibodies. Caution is required on test dose challenges though.
Table 17B.2 β-Lactam Antibiotic Skin Tests | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Because penicillin PPL and MDM are not commercially available in the United States, and skin testing with other β-lactam antibiotics has not been standardized, nor have the results been validated, test dosing (graded drug challenge) is recommended in patients with a past history of penicillin allergy. How one approaches this procedure depends on the severity of the previous reaction and the experience of the managing physician. After documenting the need for the drug, obtaining informed verbal or written consent, and being prepared to treat anaphylaxis, a graded dose challenge protocol may be initiated with a physician in constant attendance; 0.001 mg (1 unit) of the therapeutic β-lactam antibiotic is administered by the desired (oral, intravenous) route. The patient is observed for signs of pruritus, flushing, urticaria, dyspnea, and hypotension. In the absence of these signs, at 15-minute intervals, subsequent doses are given as outlined in Table 17B.3. If a reaction occurs during this procedure, it is treated with epinephrine intramuscularly and antihistamines; the need for the drug should be reevaluated and actual desensitization considered if this agent is essential. This is a rather conservative test dosing schedule. More experienced physicians may elect to shorten this procedure; one suggestion has been to test dose with 1/100 of the therapeutic dose (1/1000 of the therapeutic dose if the previous reaction was severe), and then increase toward the full therapeutic dose if there is no evidence of urticaria, flushing, wheezing, or hypotension (10).
Table 17B.3 Suggested Test Dosing Schedule for β-Lactam Antibiotics | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||
Because there is a small risk associated with skin testing and test dosing, in vitro tests have obvious appeal. Solid-phase immunoassays, such as the radioallergosorbent test (RAST) and the enzyme-linked immunosorbent assay (ELISA), have been developed to detect serum IgE antibodies against the major penicilloyl determinant. The RAST or fluorescent immunoassay generally correlates with skin testing to PPL. RAST and fluorescent immunoassays for cephalosporins and other antimicrobial drugs have been reported but are available only for research. At present, in vitro tests have limited-to-no clinical usefulness.
Management of Patients with a History of Penicillin Allergy
Preferable management of patients with a history of penicillin or other β-lactam antibiotic allergy is the use of an equally effective, non–cross-reacting antibiotic. In most situations, adequate substitutes are available, and consultation with infectious disease experts is valuable. Aztreonam, a monocyclic β-lactam antibiotic, has no clinical cross-reactivity with penicillins or cephalosporins and can be administered to patients with prior anaphylactic reactions to penicillin.
If alternative drugs fail, or if there is known antibiotic resistance by suspected pathogens, skin testing and graded dose challenge with the β-lactam antibiotic of choice should be performed. If skin tests are positive, if the patient reacts to test doses, or if such testing is not done, administration of the β-lactam antibiotic, using a graded dose challenge protocol, is advised (10,17). One begins with a subanaphylactic dose so that if anaphylaxis occurs, it can be controlled. For example, doses less than 1 mg would not be expected to induce anaphylaxis.
Some infections in which this approach becomes necessary include enterococcal infections, brain abscess, bacterial meningitis, sepsis with staphylococci, Neisseria or Pseudomonas species organisms, Listeria infections, endocarditis, osteomyelitis, neurosyphilis, and syphilis in pregnant women. In fact, penicillin desensitization is indicated for pregnant women with syphilis who demonstrate immediate hypersensitivity to that drug (10). Also, at present, there are no data to support the use of alternatives to penicillin for treatment of neurosyphilis and all stages of syphilis among HIV/AIDS-infected patients (10). With a target dose of 2,400,000 units of benzylpenicillin G, the starting dose is 0.1 unit subcutaneously, followed by 1 unit, 10 units, 100 units, 1,000 units, 10,000 units and 100,000 units. Then 200,000 units are administered intramuscularly followed by 2,100,000 units by the same route (10). This protocol delivers the 2,400,000 units for the initial dose (10).
The usual scenario involves a patient who presents with a convincing history of penicillin allergy and the physician has no available skin tests such as Pre-Pen and MDM. Therefore, graded penicillin challenge, as
previously outlined, is recommended. If a reaction occurs at any test dose, the need for the drug should be reevaluated. If essential, an actual desensitization protocol should be considered. A more unusual scenario is a patient with a positive history and available penicillin skin tests. Penicillin history–positive patients are at significant risk for anaphylaxis, but the risk can be clarified by the presence or absence of positive immediate skin tests for penicillin determinants. Desensitization protocols significantly reduce the risk for anaphylaxis in skin test–positive patients, whereas deliberate infusion of a β-lactam antibiotic at the usual rate could cause a severe or fatal anaphylactic reaction.
previously outlined, is recommended. If a reaction occurs at any test dose, the need for the drug should be reevaluated. If essential, an actual desensitization protocol should be considered. A more unusual scenario is a patient with a positive history and available penicillin skin tests. Penicillin history–positive patients are at significant risk for anaphylaxis, but the risk can be clarified by the presence or absence of positive immediate skin tests for penicillin determinants. Desensitization protocols significantly reduce the risk for anaphylaxis in skin test–positive patients, whereas deliberate infusion of a β-lactam antibiotic at the usual rate could cause a severe or fatal anaphylactic reaction.
While graded drug challenges can be performed in an outpatient facility, acute β-lactam antibiotic desensitization should be performed in a monitored or intensive care setting. Informed consent (verbal or written) is advised. Patients with asthma or congestive heart failure should be under optimal control. Premedication with antihistamines and corticosteroids is not recommended, because these drugs have not proved effective in suppressing anaphylaxis and could mask mild allergic manifestations that may have resulted in a modification of the desensitization protocol. It is believed that the early recognition of flushing and limited urticarial lesions during desensitization (or graded drug challenge) would alert the physician to the evidence of mast cell activation and risks involved. Suppression of the flushing or limited urticaria might result in a more serious, subsequent allergic reaction.
Before initiation of desensitization, two intravenous lines or one line in a large vein is established; baseline vital signs are recorded. The clinical state of the patient is assessed. A baseline electrocardiogram, spirometry have been advocated by some as well as continuous electrocardiographic monitoring depending on the patient’s comorbidities. During desensitization, vital signs and the clinical state of the patient are noted before each dose, and at 10- to 20-minute intervals following each dose. A physician must be in close attendance during the entire procedure so that unexpected reactions such as hypotension can be reversed quickly.
Desensitization has been accomplished successfully using either the oral or intravenous routes of administration (69–73). Oral desensitization is favored by some who believe that the risk for a serious reaction is less. The intravenous route is chosen by others, including myself, who prefer absolute control of the drug concentration used and its rate of administration. Unfortunately, there is no completely standardized regimen, and there have been no direct comparative studies between oral and intravenous desensitizing protocols.
Regardless of the method chosen for desensitization, the basic principles are similar. The initial dose is typically
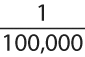 of the therapeutic dose. Oral desensitization may begin with the dose that is tolerated during oral test dosing. Intravenous desensitization should begin with 1/10 or 1/100 (if the previous reaction was severe) of the dose producing a positive skin test or intravenous test dose response. The dose is then usually doubled at 7- to 15-minute intervals until full therapeutic doses are achieved, typically within 4 to 5 hours. Representative protocols for intravenous (Table 17B.4) and oral (Table 17B.5) desensitization are presented.
of the therapeutic dose. Oral desensitization may begin with the dose that is tolerated during oral test dosing. Intravenous desensitization should begin with 1/10 or 1/100 (if the previous reaction was severe) of the dose producing a positive skin test or intravenous test dose response. The dose is then usually doubled at 7- to 15-minute intervals until full therapeutic doses are achieved, typically within 4 to 5 hours. Representative protocols for intravenous (Table 17B.4) and oral (Table 17B.5) desensitization are presented.
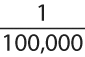
Table 17B.4 Protocol for Intravenous Desensitization with β-Lactam Antibiotics | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Table 17B.5 Protocol for Oral Desensitization with β-Lactam Antibiotics | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Table 17B.4 outlines an intravenous desensitization protocol for penicillin G potassium or any other β-lactam antibiotic (10). The dose to be administered is placed in a small volume of 5% dextrose in water for piggyback delivery into the already established intravenous line. It is administered slowly at first, then more rapidly if no warning signs, such as pruritus or flushing, appear. If symptoms develop during the procedure, the flow rate is slowed or stopped and the patient treated appropriately, using the other intravenous site if necessary. After symptoms subside, the flow rate is slowly increased once again. Once the patient has received and tolerated 800,000 units of penicillin G or 800 mg of any other β-lactam antibiotic, the full therapeutic dose may be given and therapy continued without interruption.
Table 17B.5 provides a protocol for oral desensitization with β-lactam antibiotics. If the patient is unable to take oral medication, it may be administered through a feeding tube. Mild reactions during desensitization, such as pruritus, fleeting urticaria, mild rhinitis, or wheezing, require the dose to be repeated until tolerated. If a more serious reaction occurs, such as hypotension, laryngeal edema, or severe asthma, the next dose should be decreased to at least one-third of the provoking dose and withheld until the patient is stable. If an oral form of the desired β-lactam agent is unavailable, intravenous desensitization should be considered. Once desensitized, treatment must not lapse. Regardless of the route selected for desensitization, mild reactions, usually pruritic rashes, may be expected in about 0 to 30% of patients during and after the procedure. These reactions usually subside with continued treatment, but symptomatic therapy may be necessary.
After successful desensitization, some individuals may have predictable needs for future exposures to β-lactam antibiotics. Patients with cystic fibrosis, chronic neutropenia, or occupational exposure to these agents may benefit from chronic twice-daily oral penicillin therapy to sustain a desensitized state between courses of high-dose parenteral therapy (71). However, some investigators are concerned about the ability to maintain 100% compliance among cystic fibrosis patients in an outpatient setting and therefore prefer to perform intravenous desensitization each time β-lactam antibiotic therapy is required (72).
In summary, β-lactam antibiotics can be administered by desensitization with relatively little risk in most patients with a history of allergy to these drugs and a positive reaction to skin testing. Once successfully desensitized, the need for uninterrupted therapy is advisable until treatment has been completed. Any lapse in therapy greater than 12 hours may permit such sensitivity to return. Mild reactions during and after desensitization are not an indication to discontinue treatment. Many such reactions resolve spontaneously or may require symptomatic therapy.
Among successfully desensitized patients with a positive history of β-lactam allergy and a positive response to skin testing or graded challenge, this same approach may be repeated before a future course of therapy. There appears to be little risk for resensitization following an uneventful course of therapy among patients with positive histories and negative skin tests or after uneventful test dosing (67,68). Desensitization does require an essential indication for the incriminated antibiotic (74,75) and preparedness for anaphylaxis and its treatment. In the absence of skin testing, which helps to place the patient at high risk (if positive) and very low risk (if negative), patients with penicillin allergy are often undergoing graded drug challenges as opposed to actual desensitization. Nevertheless, when beginning the graded challenge, without skin test results, the risk is based on the history and patient’s co-morbidities, including ineffectively controlled asthma or sepsis, as opposed to more precise data such as presence or absence of antipenicillin major determinant, Pre-Pen, or MDM, IgE antibodies.
Non–β-Lactam Antimicrobial Agents
Allergic reactions to non–β-lactam antimicrobial drugs, most commonly cutaneous eruptions, are common causes of morbidity and, rarely, mortality. Anaphylaxis to these agents is a rare event. The estimated overall incidence of a hypersensitivity-type reaction to
non–β-lactam drugs is about 1% to 3%. Some antimicrobial agents, however, such as TMP-SMX, produce reactions more commonly; in contrast, others, such as tetracycline, are much less likely to do so.
non–β-lactam drugs is about 1% to 3%. Some antimicrobial agents, however, such as TMP-SMX, produce reactions more commonly; in contrast, others, such as tetracycline, are much less likely to do so.
Unlike the β-lactam antimicrobials, other antibiotics have been less well studied and also include a wide variety of chemical agents. Research has been hampered by the lack of information regarding the immunochemistry of most of these drugs and, therefore, the unavailability of proven immunodiagnostic tests to assist the physician. Although skin testing with the free drug and some in vitro tests have been described for sulfonamides, aminoglycosides, and vancomycin, there are no large series reported to validate their clinical usefulness. Use of pharmacogenomic data prospectively should permit more precise “personalized medicine” and result in fewer adverse reactions.
Despite these shortcomings, when such agents, notably TMP-SMX, are medically necessary, protocols have been developed to administer these drugs (4,10,16). With the exception of sulfonamides and occasionally other non–β-lactam drugs, urgent administration is usually not required. Slow, cautious test dosing is generally a safe and effective method to determine whether the drug is now tolerated. An example is with TMP-SMX, where one can use the suspension containing 40 mg trimethoprim and 200 mg sulfamethoxazole per 5 mL (4,10,16). The first dose is with 0.1 mg orally of the dose for sulfamethoxazole and, at 30- to 60-minute intervals, administer 1 mg, 10 mg, and 50 mg. If there is no reaction, on the following day, 100 mg and 200 mg may be given. On occasion, particularly in life-threatening Pneumocystis or toxoplasma infections in HIV/AIDS patients, an every-4-hour dosing schedule may be required. Because most reactions to non–β-lactam antimicrobial agents are nonanaphylactic (IgE-independent), desensitization is indicated rarely and may be quite dangerous, as described later.
Sulfonamides
Background
The stimulus for continued attention to sulfonamide and trimethoprim hypersensitivity is due to their utility in treatment of a wide variety of gram-positive and gram-negative bacterial infections and to their importance in the acute or empiric treatment of infectious complications in patients with HIV/AIDS. In patients
infected with HIV and living in poor countries, TMP-SMX may be used as prophylaxis and primary therapy for Pneumocystis pneumonia, and as prophylaxis for Toxoplasma gondii infections, and as treatment for Isospora belli gastroenteritis. The combination of sulfadiazine and pyrimethamine is available for treatment of chorioretinitis and encephalitis due to toxoplasmosis in HIV-positive patients. Another sulfonamide, sulfasalazine, may be used in the management of inflammatory bowel disease, although the alternatives, olsalazine and mesalamine are considerably safer alternatives.
infected with HIV and living in poor countries, TMP-SMX may be used as prophylaxis and primary therapy for Pneumocystis pneumonia, and as prophylaxis for Toxoplasma gondii infections, and as treatment for Isospora belli gastroenteritis. The combination of sulfadiazine and pyrimethamine is available for treatment of chorioretinitis and encephalitis due to toxoplasmosis in HIV-positive patients. Another sulfonamide, sulfasalazine, may be used in the management of inflammatory bowel disease, although the alternatives, olsalazine and mesalamine are considerably safer alternatives.
The most common reaction ascribed to sulfonamide hypersensitivity is a generalized rash, usually maculopapular in nature, developing 7 to 12 days after initiation of treatment. Fever may be associated with the rash. Urticaria is occasionally present, but anaphylaxis is a rare event. The TMP-SMX may have been associated with acute urticaria or other immediate reaction; while it is often considered to be from SMX, anaphylaxis and allergic reactions have been attributable to TMP (76–79). In addition, severe cutaneous reactions, such as Stevens-Johnson syndrome and toxic epidermal necrolysis, may occur. Hematologic reactions, notably thrombocytopenia and neutropenia, serum sickness–like reactions, as well as hepatic and renal complications may occur occasionally.
Diagnostic Testing
There are no in vivo or in vitro tests available to evaluate the presence of sulfonamide allergy. However, there is evidence that some of these reactions are mediated by an IgE antibody directed against its immunogenic metabolite, N4-sulfonamidoyl (80). Further, studies using multiple N4-sulfonamidoyl residues attached to polytyrosine carrier as a skin test reagent have been reported (81), but additional studies are necessary to evaluate its clinical usefulness. Also, it appears that most sulfonamide reactions are not IgE-mediated. One notion is that most adverse reactions are due to hydroxylamine metabolites, which induce in vitro cytotoxic reactions in peripheral blood lymphocytes of patients with sulfonamide hypersensitivity (82–84). Pharmacogenetics explain some adverse reactions as there are wide variations in acetylation, e.g., slow acetylators experiencing more adverse reactions. The enzyme arylamine N-acetyltransferase 2 (NAT2) has multiple polymorphisms that account for variations in acetylation status (85). From 45% to 70% of sulfamethoxazole is acetylated to N-acetylsulfamethoxazole, with little oxidized to hydroxylamine (86




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree