Parasitic infections include diseases caused by protozoa or helminths. These infections have a worldwide distribution with an estimated 30% of the world’s population experiencing parasitic infestations. In addition, significant parasitic infestations occur during pregnancy. These parasitic infections are generally more common in tropical and underdeveloped areas of the world where they account for a large burden of morbidity and mortality. The protozoan organisms Plasmodium sp. (malaria), Entamoeba histolytica, Trypanosoma sp., and Leishmania sp., are major causes of disease and mortality in Africa, Asia, Central and South America. Malaria affects 250 million people and is responsible for up to 2 million deaths annually. Helminths are the most prevalent infectious agents in humans. In the recent past, Ascaris, Trichuris, and the hookworms accounted for nearly one billion infections each, whereas the schistosomes and filariae accounted for 250 million infections each.
As the result of substantial international cooperation, partnerships with pharmaceutical companies, introduction of new diagnostic techniques, availability of new patient treatment, and vector control, the prevalence of several parasitic infections (e.g., lymphatic filariasis, Chagas disease, dracunculiasis, and onchocerciasis) have decreased substantially to levels suggesting these diseases may soon be eliminated. Unfortunately, other parasitic diseases have re-emerged because of inadequate intervention and control strategies and the breakdown of healthcare delivery systems. These include leishmaniasis, malaria, African trypanosomiasis, and helminthic infection.
The Millennium Declaration adopted by the United Nations in 2000 established eight development goals to eliminate extreme poverty, hunger, and disease by 2015. The sixth goal addressed the health and economic impact of infectious diseases with the objective, “to combat human immunodeficiency virus infection (HIV)-AIDS, malaria, and other diseases.” While large-scale well financed (public and private) programs have been initiated to combat HIV-AIDS, malaria, and tuberculosis, many of the “other diseases” have not benefited from similar attention. These “other diseases” are collectively referred to as the “Neglected Tropical Diseases.” These neglected tropical diseases include 13 parasitic and bacterial infections
(Table 22.1).The range of protozoans and helminths that infest humans is vast.
Table 22.2 lists those infestations which are either common or have a potentially adverse effect on pregnancy outcome. While the prevalence of parasitic diseases during pregnancy is much lower in the United States and other Western industrialized nations, the wide accessibility to rapid foreign travel has resulted in a rather large “at risk” pool of tourists exposed to a multitude of protozoan and helminth infections during their travels. Ironically, the response to parasitic infection by nonresident visitors to endemic areas may be more severe than that of the local inhabitants who have acquired immunity to these agents. The recent influx of immigrants into the United States from South and Central America and Southeast Asia has led to the presence of a population with a very high prevalence of parasitic infestation. In addition, the use of immunosuppressive drugs and the spread of HIV infection have led to an increased prevalence of infections caused by a variety of parasites. Finally, there are areas in the United States where the environmental, economic, and sanitary conditions are appropriate for the maintenance of endemic parasites. Epidemics of giardiasis and amebiasis have occurred in the United States, schistosomiasis is endemic in Puerto Rico, and hookworm is endemic in the Southeastern United States.
Entamoeba histolytica is a frequent cause of diarrhea in HIV-infected men who have sex with men (MSM).
Parasitic infestation may adversely affect fertility and/or reproductive capacity in three ways
(see Table 22.3). First, the infecting organism can result in sufficient debilitation and/or anatomic damage to the genital tract so that either conception is impossible or normal implantation does not occur. Second, parasitic infestations may be severe enough to adversely affect the mother’s health to the point where medical intervention to terminate the pregnancy is required. Third, protozoan parasites may infect and cross the placenta to produce adverse fetal effects including abortion, fetal infection, stillbirth, intrauterine growth restriction, and congenital infection. Additional mechanisms for producing adverse effects on pregnancy outcome have been proposed. The nutritional status of pregnant women in the tropics and underdeveloped areas is borderline. Parasitic disease may significantly interfere with the nutrition of these women and may result in a worsening of the already critical nutritional status with resultant impaired fetal growth. Another factor leading to poor outcome may be that malnutrition is associated with immunodeficiency, and thus the susceptibility of pregnant women to bacterial and viral infections and their recognized consequences for the fetus and newborn is increased.
In general, the majority of parasitic infections that occur in pregnancy without HIV infection do not require treatment during pregnancy. However, if severe symptoms, anemia, and/or malabsorption occur, treatment should be initiated during pregnancy. As discussed later, certain parasitic infections should always be treated during pregnancy. These include symptomatic amebiasis, severe giardiasis, malaria, and ascariasis. Moreover, parasitic infections associated with HIV infection such as Pneumocystis carinii and Toxoplasma gondii should be aggressively treated.
Because of the above mentioned levels of worldwide travel, immigration patterns and spread of HIV infection, health-care providers should be cognizant of protozoan and helminthic infections. Diagnosis of these infections presenting
in the United States and other industrialized countries is dependent on physicians considering the possibility of parasitic disease. The key to recognition of these infections includes knowledge of epidemiologic risk factors, geographic distribution and clinical presentation. In addition, diagnosis and treatment require use of tests and drugs which are unfamiliar to most physicians in the U.S. These issues will be discussed in the remainder of this chapter.
PROTOZOAN INFECTIONS
Amebiasis
Amebiasis is an ulcerative and inflammatory disease of the colon caused by the protozoan Entamoeba histolytica. The organism may also occur in extraintestinal sites, most commonly, the liver where severe tissue damage can occur. Amebiasis occurs throughout the world, with its prevalence being highest in tropical areas and countries with low levels of sanitation and personal hygiene. Entamoeba histolytica is the pathogenic strain which can invade tissue and produce symptomatic disease. An estimated 10% of the world’s population is infected with nearly 50% of the population infected in many developing countries.
Each year in the world there are approximately 50 million cases of symptomatic diseases with an estimated 100,000 deaths. In the United States the overall prevalence of Entamoeba infection is estimated to be 3%-4%. However, a high incidence of infection is present in certain high-risk groups (e.g., institutionalized populations, sexually promiscuous MSM, recent immigrants or migrant workers from E. histolytica endemic areas). E. histolytica is the third most common cause of death from parasitic infections in developing nations and a major health risk to travelers to these areas. E. histolytica is not transmitted across the placenta and thus does not have a direct effect on the fetus. However, it can produce severe maternal disease during pregnancy or in association with malnutrition.
Infection is acquired by ingestion of the cyst form of E. histolytica in food or water contaminated by feces. Direct fecal-oral contact also transmits the cyst form. The cysts rupture in the small bowel to release the trophozoites. The trophozoites migrate to the colon. If environmental conditions are not appropriate for continued multiplication, encystment occurs, and the life cycle is complete. Following encystment of a trophozoite the cysts are excreted in feces and can survive for weeks in an appropriate moist environment.
The trophozoite, under the influence of poorly understood environmental factors, may invade the mucosal wall of the
colon. Trophozoites are facultative anaerobes, and once they gain access to submucosal areas, they are capable of maintaining an ideal environment for their survival by producing tissue anoxia, necrosis, and a low pH. It is this large area of necrosis in the submucosa that produces the characteristic flask-shaped ulcer of amebiasis. Hepatic infection occurs when trophozoites ascend in the portal venous system and produce hepatic necrosis secondary to obstruction of portal vessels.
Clinical Presentation
Clinical findings associated with E. histolytica infestation range from the asymptomatic carrier state to fulminant dysentery. Clinical disease may occur in 10% to 50% of asymptomatic carriers. Immunosuppression, malnutrition, steroid therapy, and pregnancy have been associated with development of clinical disease in asymptomatic carriers. In addition, children (especially neonates) are at risk for development of symptomatic disease.
Clinical amebiasis may be mild (noninvasive disease) and characterized by colonic irritation with colicky lower abdominal pain and increased frequency of bowel movements; stool may be loose with mucus and/or blood present. With more extensive colonic disease (invasive), the patient presents with bloody frequent diarrhea stools, abdominal pain and tenderness, right upper quadrant pain and tenderness, and hepatomegaly. Acute amebic rectocolitis (dysentery) is characterized by gradual onset over 1 to 3 weeks, abdominal pain, diarrhea, dysentery, weight loss, fever (one third of cases), abdominal tenderness, and heme-positive stools. The reported case fatality rate ranges from 2% to 9% with some studies noting that the case fatality rate in women was twice that of males. Fulminant colitis is an infrequent but highly lethal form of amebic infection. It has a predilection for malnourished individuals, pregnant women, patients on corticosteroids and young infants. These patients present with severe illness characterized by fever, leukocytosis, profuse bloody mucoid diarrhea, diffuse abdominal pain and often hypotension with signs of peritonitis following transmural necrosis of the bowel. In the extreme, an acute abdomen can be present secondary to bowel perforation and acute amebic peritonitis following transmural necrosis of the bowel. This serious complication occurs in 3% to 4% of patients with severe amebic dysentery and is associated with a high mortality rate.
The majority of patients with amebic liver abscesses have no history of antecedent or current bowel disease. Patients with amebic liver abscess present with acute onset (<10 days) of right upper quadrant pain, fever, tender hepatomegaly, rapid weight loss, and pallor. Alternatively, liver abscess may present subacutely with weight loss the most prominent finding and less than 50% of patients having fever or abdominal pain. Amebic liver abscesses may extend into adjacent organs, most commonly the pleural cavity, but rupture into the pericardium or peritoneum may also occur.
Cerebral amebiasis is a rare cause of brain abscess. However, its onset is abrupt with rapid progression to death within 12 to 72 hours unless appropriate therapy is commenced. Genitourinary amebiasis is also very rare and is found in association with rectovaginal fistulas which allow spread of E. histolytica trophozoites to the genital tract. Genitourinary amebiasis presents as painful granulation tissue or ulcers mimicking malignancy.
Diagnosis
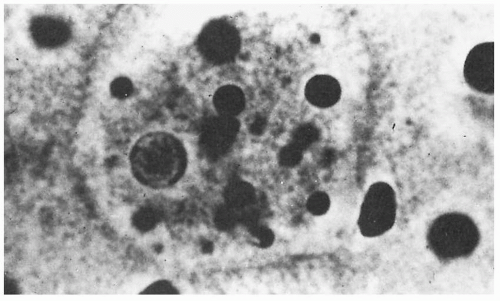
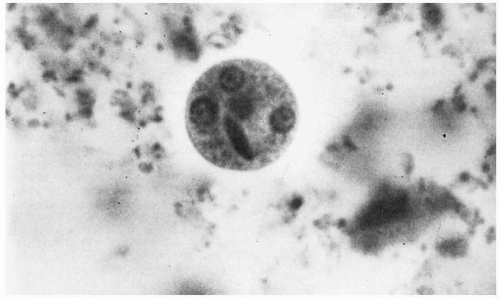
The diagnosis of intestinal amebiasis is most commonly made by demonstrating
E. histolytica in the stool. Either the presence of trophozoites or cysts confirms the diagnosis
(Figs. 22.1 and 22.2). Stool specimens are best examined fresh. Examination of a single stool specimen identifies only one third of infected patients. Thus three specimens should be assessed before excluding the diagnosis of amebiasis. While stool cultures for
E. histolytica are much more sensitive they are not usually clinically available. Endoscopy is an excellent approach for making the diagnosis. Amebic colitis appears as punctuate hemorrhagic areas or small ulcers with exudative centers and hyperemic borders. Aspiration of these lesions provides a reliable specimen for identification of motile, erythrocyte-containing trophozoites of
E. histolytica.Direct detection of E. histolytica antigen in serum or feces and serum anti-amebic antibody tests has been shown to be useful in the diagnosis of invasive intestinal amebiasis. Most recently, polymerase chain reaction (PCR), based on amplification of an RNA gene, is available to identify E. histolytica in stool specimens and from liver abscesses.
Patients with amebic liver abscess often do not have concurrent intestinal involvement, and thus, stool specimens are negative for E. histolytica. Diagnosis of amebic liver abscess relies on recognition of the clinical manifestations and epidemiologic risk factors, noninvasive imaging studies demonstrating a solitary (usually) defect in the right lobe of the liver and detection of serum anti-amebic antibodies. Imaging techniques include ultrasonography, CT, magnetic resonance imaging (MRI), and Gallium scan. None are specific for amebic liver abscess. Ultrasound is rapid and less costly and thus should be the initial imaging technique. In general, aspiration of the cyst is not necessary to make the diagnosis.
Effects of Amebiasis on Pregnancy
Several reports have suggested that amebiasis during pregnancy may be more severe and may be associated with a higher mortality rate than occurs in nonpregnant women. Up to 68% of fatal cases of amebiasis in females occur in association with pregnancy. Factors that may account for this increased susceptibility during pregnancy include: (i) increased levels of free plasma cortisol during pregnancy; (ii) increased levels of serum cholesterol in early pregnancy; and (iii) malnutrition and anemia, which are commonly present in pregnant women living in areas endemic for amebiasis.
It has also been suggested that amebiasis during pregnancy may result in an adverse effect on the fetus. An increased incidence of amebiasis in women with spontaneous abortion, stillbirths, preterm deliveries, and infants having congenital anomalies compared with normal term deliveries has been reported. It is important to recognize that asymptomatic or mild amebiasis may develop into severe amebic dysentery during pregnancy and the puerperium. Thus, consideration should be given to treatment of these milder forms of amebiasis in pregnant women.
There is no evidence that E. histolytica is associated with intrauterine fetal infection. However, the newborn may acquire the disease secondary to person-to-person transmission, usually from its mother. Neonates with amebiasis present with sudden onset and are seriously ill with bloody diarrhea, hepatic abscess, gangrene of the colon, and colon perforation with peritonitis.
Treatment
It is recommended that even asymptomatic infection with
E. histolytica be treated. Because the cyst stage of
E. histolytica is
resistant to physical and chemical agents, antimicrobial therapy is directed against the trophozoite stage. A variety of drugs are available, and the treatment regimen should be based on both the location (intraluminal, intestinal, submucosa and/or extraintestinal) and severity of amebiasis. In addition, it is very important that pregnant women with amebiasis be treated during pregnancy because of the reported increased incidence of severe disease in pregnancy.
Table 22.4 summarizes the recommendations for treatment of amebiasis. Paromomycin (Humatin) is the drug of choice, in pregnancy, for asymptomatic disease. Metronidazole 750 mg t.i.d. for 10 days is an alternative choice for pregnancy. Diloxanide furate is not commercially available in the United States but can be obtained from the CDC.
Symptomatic intestinal and extraintestinal amebiasis requires treatment with an agent active against trophozoites invading the colon wall or extraintestinal sites followed by an agent able to eradicate intraluminal encysted E. histolytica (paromomycin or iodoquinol).
Giardiasis
Giardia lamblia is a flagellated protozoan parasite that has a cystic stage which infects the host and a motile trophozoite stage which produces disease. It is a frequent cause of endemic and epidemic diarrhea. Giardiasis is a ubiquitous disease with worldwide distribution. However, its incidence is higher in developing countries where poverty, poor sanitation, lack of medical care and overcrowding facilitate the spread and persistence of the organism. Areas of increased risk include Southeast and South Asia, West and Central Africa, South America, Mexico, Korea, and the Soviet Union. Waterborne outbreaks of Giardiasis frequently occur in mountainous areas of the northeast, northwest, and Rocky Mountain states in the United States. These outbreaks are generally caused by faulty purification systems or untreated water in wilderness areas. G. lamblia is the most commonly identified pathogenic intestinal parasite in the United States, occurring in 3% to 9% of stool specimens.
The resistant cyst stage is transmitted by the fecal-oral route. Thus acquisition of G. lamblia requires oral ingestion of fecally contaminated water. Person-to-person transmission is also frequent in daycare centers and among men having sex with men. With exposure to the acidic pH of the stomach, the cysts rupture to release trophozoites. The trophozoites multiply rapidly in the small bowel and firmly attach via their suckling disc to the intestinal epithelium. The mechanism for the diarrhea and occasionally malabsorption is caused by a complex interaction between Giardia and the host related to number and genotype of Giardia ingested and the host’s prior exposure to and immune response to the organism.
Clinical Presentation
Giardiasis may range from an asymptomatic carrier state to severe diarrhea with malabsorption. Of persons ingesting
G. lamblia 5% to 15% become asymptomatic cyst passers, 25% to 50% become symptomatic with an acute diarrheal syndrome, and 35% to 70% do not have any signs or symptoms of infection. Following ingestion of
G. lamblia there is an incubation time of 1 to 2 weeks prior to onset of symptoms. Characteristically, giardiasis presents with sudden onset of explosive, watery, foul-smelling, bulky diarrhea. Anorexia, nausea, vomiting, belching of sulfuric material, abdominal cramps, low-grade fever, chills, and malaise also occur. There is an absence of blood, mucus, or polymorphonuclear cells in the stool. Most cases last for several weeks. In most instances, giardiasis runs a relatively benign course. However, children
<5 years old and pregnant women may present with severe illness and hypovolemia requiring hospitalization.
Diagnosis
It is important to consider a diagnosis of giardiasis in any patient presenting with prolonged diarrhea, especially when associated with weight loss or malabsorption. Previously, microscopy of stool for ova and parasites was the gold standard for diagnosis of giardiasis. In the early acute stage, giardiasis can be diagnosed by immediate examination of a stool wet smear which demonstrates trophozoites. Later in the disease process, the stool is more formed and contains the cyst form. Concentration techniques are usually necessary to demonstrate these cysts. Recently commercially available tests for rapid detection of Giardia antigen in the stool have become available. These tests have high sensitivity and specificity and are superior to stool exam for ova and parasites. These include detection of Giardia antigen by immunofluorescence on enzyme-linked absorbent assay. A nonenzymatic, solid phase assay has also been developed. In difficult cases sampling of duodenal contents by aspiration or biopsy may be required to demonstrate motile trophozoites.
Effect on Pregnancy
In general, giardiasis has minimal adverse effects on pregnancy outcome. However, significant malabsorption may impair fertility and adversely affect pregnancy. Studies describing severe giardiasis in pregnant women suggest that the disease may be more severe in pregnancy with significant weight loss and debility.
Treatment
Patients with symptomatic giardiasis should be treated
(Table 22.5). While not FDA approved for use in giardiasis, metronidazole has been shown to be both effective and safe with extensive clinical experience. Recently, tinidazole (Tindamax, Presutti Labs) and nitazoxanide (Alinia, Romark) have become available in the United States and are FDA approved for the treatment of giardiasis.
Treatment of giardiasis in pregnancy has been somewhat controversial because of theoretic concerns with the use of metronidazole in pregnancy. However, the safety of metronidazole in pregnancy has been widely studied and consensus has emerged that it is safe to use in pregnancy, even in the first trimester. Tinidazole is a Pregnancy Category C drug. Paromomycin is an alternative for pregnancy as it is nonabsorbable. For pregnant women with mild disease in the absence of dehydration or malnutrition, treatment can be delayed until after delivery or at least until after the first trimester.
LEISHMANIASIS
Leishmaniasis is a group of clinical diseases produced by protozoan organisms of the genus Leishmania. It is endemic in many subtropical regions of the world. Leishmaniasis has grown in importance because of the widespread accessibility of international travel and the AIDS epidemic.
Leishmanias exist as intracellular amastigotes in macrophages in humans and other mammalian hosts and as extracellular promastigotes in the gut of their insect vectors, phlebotomine sand flies. The six major species and their subspecies which are human pathogens are listed in
Table 22.6. These various species of
Leishmania are transmitted by the bite of infected female sandflies. Rarely, transmission may occur in utero or at the time of birth. A single species of
Leishmania can present as different clinical syndromes and each syndrome can be caused by more than one species. Other than New World cutaneous leishmaniasis where differentiating
L. mexicana group from
L. vianna group has treatment implications, recognition of the clinical forms of Leishmaniasis is the key to appropriate therapy.
The clinical manifestations of leishmaniasis vary as a result of the virulence of the infecting species and the cell-mediated immune response of the host. Leishmaniasis has been divided into three distinct clinical syndromes. Kala-azar (visceral or systemic leishmaniasis) is usually caused by L. donovani, L, infantum, or L. chagosi. Cutaneous leishmaniasis (oriental sores) in the Old World is produced by L. tropica, L. aethiopaica, and L. major. When widespread cutaneous papules or nodules are present all over the body, the condition is labeled “diffuse cutaneous leishmaniasis.” American leishmaniasis (mucocutaneous disease) is caused by L. mexicana and L. viannia group. The L.(V.) braziliensis complex is responsible for mucosal disease in the Americas.
Visceral leishmaniasis is widely distributed throughout the world, with its greatest prevalence in India, Bangladesh, and northeastern Brazil. Cutaneous leishmaniasis occurs in tropical and subtropical areas of the Eastern and Western hemispheres, especially the Middle East, Asia, Central and South America, and the Indian subcontinent. The mucocutaneous form of the disease is endemic in Central and South America.
According to WHO, an estimated 350 million people are at risk for leishmaniasis worldwide. The incidence of cutaneous disease is estimated to be 1 to 1.5 million cases annually and that of visceral leishmaniasis to be 500,000 cases annually.
Clinical Presentation
Visceral leishmaniasis (Kala-azar) has a slow insidious onset following an incubation period of 3-8 months. It characteristically presents as prolonged fever, progressive weight loss,
weakness, hepatosplenomegaly, anemia, leukopenia, hypoalbuminemia, and hyperglobulinemia. As the disease progresses the skin becomes grayish in color. It is this discoloration that led to the name kala-azar which is the Indian word for “black fever.” Untreated visceral leishmaniasis may be fatal. This form of leishmaniasis is an important opportunistic infection in persons with HIV infection in Spain, France, and Italy.
Old-World cutaneous leishmaniasis has an incubation period ranging from two weeks to several months. With L. tropica (urban or dry form) the lesions are single, grow slowly and persist for ≥1 year. In disease caused by L. major and L. braziliensis (rural or moist form) lesions may be multiple, have a granulating base, grow rapidly and heal within several months. Cutaneous leishmaniasis initially appears as a pruritic red papule that occurs at the site of inoculation. This lesion slowly progresses to a shallow ulcer with seropurulent discharge. Tender regional lymphadenopathy is present. The ulcer generally heals spontaneously over several months. Diffuse cutaneous leishmaniasis begins as a localized papule but does not ulcerate. Rather satellite lesions develop and organisms spread to distant sites on the skin, especially the face and extremities. This form progresses very slowly and can persist for 20 years or more.
In American cutaneous leishmaniasis the incubation time is 2 to 8 weeks. A wide variety of skin lesions may present, ranging from small dry, crusted lesions to large mutilating ulcers. Lesions may be single or multiple and occur primarily on exposed areas of the body. L. braziliensis can persist after resolution of the primary cutaneous ulcer resulting in mutilating infection involving mucosal areas.
While the mucocutaneous form of leishmaniasis begins similarly as a small papule which progresses to an ulcerative stage, this disease is progressive in nature, with development of new ulcerations involving the mucocutaneous borders of the nose and mouth. Initial symptoms include nasal congestion, discharge, discomfort or epistaxis. Extensive tissue destruction and scarring occur, as these lesions slowly heal over several years. The nasal septum may be destroyed leading to nasal collapse or perforation can occur through the soft palate. Involvement of the genital mucosa has been reported.
Diagnosis
The diagnosis of leishmaniasis may be difficult because of the various forms of the disease, the number of Leishmania species involved, geographic variations and other syndromes with similar clinical manifestations. Diagnosis of leishmaniasis should always be considered in known endemic areas of the world. Unfortunately, in the United States the diagnosis is often delayed or missed in immigrants or returning international travelers. Indigenous cutaneous leishmaniasis occurs in the U.S. in a region of Southern Texas extending from San Antonio to the Mexican border.
The gold standard for diagnosis of leishmaniasis is the isolation or identification of the causative parasite from infected sites. Molecular probes are available in research studies, but in clinical practice the diagnosis of leishmaniasis is most often confirmed by identification of leishmania amastigotes in a Wright-Giemsa-stained slide preparation or tissue specimen. In addition, leishmania may be isolated with culture media (obtained from the CDC in the United States). Speciation can be determined at WHO reference laboratories utilizing isoenzyme patterns, monoclonal antibodies, and DNA hybridization with or without PCR. In immunocompetent persons, anti-leishmanial antibodies are usually present in high titers with the use of ELISAs, indirect immunofluorescence assays or direct agglutination tests. Assays based on recombinant leishmanial antigens have recently been developed which are more specific. The diagnosis of visceral leishmaniasis (kala-azar) is suggested by the characteristic clinical presentation occurring in an endemic area and is confirmed by demonstrating leishmania bodies within cells or the reticuloendothelial system, usually by marrow aspiration or splenic aspiration.
Cutaneous leishmaniasis is suspected when the typical ulcer appears in an endemic area. The diagnosis is confirmed by demonstrating the organism (amastigotes) in smears or promastigotes in cultures obtained from the borders of the lesion. The Montenegro skin test becomes positive during the course of this form of leishmaniasis. Anti-leishmanial antibodies may be present in some patients with cutaneous leishmaniasis but the titers are usually low.
Mucosal leishmaniasis is diagnosed definitively by identifying amastigotes in touch preparations or tissue specimens or isolating promastigotes in culture. The causative agent of mucosal disease, L. (V.) braziliensis, is difficult to grow and the parasite burden is low. Thus the diagnosis is often made presumptively based on the clinical findings, a characteristic scar evidencing prior skin infection and either a positive leishmanin skin test (Montenegro) or anti-leishmanial antibodies.
Effect on Pregnancy
Visceral leishmaniasis (Kala-azar), caused by L. donovani, is the only form of leishmaniasis in which a parasitemia occurs. Consequently, it is the only leishmaniasis that has been reported to cause intrauterine fetal infection. Mother-to-child transmission of visceral leishmaniasis has been suggested by the occurrence of congenital infection in newborns. Visceral leishmaniasis (kala-azar) acquired during pregnancy has been associated with increased fetal loss. Pregnant women with visceral leishmaniasis require treatment. In a recent report from Brazil, cutaneous leishmaniasis during pregnancy was noted to produce distinct lesions which were larger than in nonpregnant patients and exophytic. High rates of preterm births (10.5%) and stillbirths (10.5%) were reported.
Treatment
The treatment of leishmaniasis depends on the infecting species, the clinical syndrome, the immunologic status of the patient, and the availability and cost of drugs. Traditionally pentavalent antimony-containing compounds were considered the drugs of choice. However, increasing drug resistance and treatment failures, failure of immunocompromised patients to respond and frequent side effects are associated with use of these agents. For visceral leishmaniasis (kala-azar) liposomal amphotericin B is the only drug currently approved by the U.S. Food and Drug Administration (FDA). It is as effective as and is less toxic than pentavalent antimony. However, it is expensive. The recommended dosing for immunocompetent and immunocompromised patients is provided in
Table 22.7. The high cost of liposomal amphotericin B has largely limited its use to the United States and other industrialized countries. In
many areas of the world (because of cost) pentavalent antimony remains the drug of choice for initial treatment of visceral leishmaniasis. In the United States and other English-speaking countries stibogluconate sodium (pentostam) which contains approximately 100 mg/mL of pentavalent antimony is available (through the Centers for Disease Control). Side effects are common with pentavalent antimony therapy. More recently miltefosine, an oral agent, is being used in India for visceral leishmania because of widespread resistance to pentavalent antimonial drugs. Miltefosine has potent reproductive toxicity and can not be used in pregnancy. Alternatives include pentamidine isethionate and amphotericin B deoxycholate, agents as effective but more toxic.
With cutaneous leishmaniasis, the goal of therapy is to limit tissue damage and with infection caused by L. braziliensis to prevent subsequent development of American mucosal disease. The decision whether to treat or not to treat cutaneous leishmaniasis depends on the location and extent of lesion(s) and on the species involved. In areas of the world without mucosal disease and with lesions healing or located at a “cosmetically insignificant site,” cutaneous leishmaniasis can be followed without therapy or treated topically unless infection is caused by L. braziliensis. Large or disfiguring lesions should be treated.
For the treatment of cutaneous leishmaniasis, the drug of choice is stibogluconate sodium (Pentostam). Treatment may be continued or repeated until there is a response. Imidazole antifungals (e.g., ketoconazole, itraconazole, and flucoconazole) have been successfully used as well. Experience with miltefosine is limited to date. A combination of pentavalent antimony and concurrent interferon-gamma has been successful in treating diffuse cutaneous leishmaniasis, a form of the disease which responds poorly to antimony only. Cutaneous disease caused by L. major may be treated with topical paromomycin 15% twice daily for 15 days. Recently, topical imiquimod 5% concomitant with antimony therapy was reported to be effective. Treatment of cutaneous disease in pregnancy is generally not necessary unless infected with L. braziliensis or if lesions involve the face.
Mucosal leishmaniasis caused by L. braziliensis is less responsive to pentavalent antimony. In patients unresponsive to or relapsing after antimony therapy, amphotericin B 0.5 to 1.0 mg/kg IV daily or every other day (total dose, 1.5-2.0 g) should be given. Pentamidine isethionate 2 to 4 mg/kg once or twice weekly is an alternative choice.
The experience with anti-leishmania drugs in pregnancy is limited, and their effects are not clear. They should be used with caution, and only treatment of visceral leishmaniasis (kala-azar) seems appropriate during pregnancy.
CHAGAS DISEASE (AMERICAN TRYPANOSOMIASIS)
Chagas disease is caused by T. cruzi and occurs in Central and South America and Mexico, where millions are infected. T. cruzi is transmitted by blood sucking triatomine insects (kissing bugs), which live in the cracks and holes of primitive dwellings in urban shanty towns. This insect vector is found in the Americas from the Southern United States to Southern Argentina. It is estimated that 10 to 12 million people are infected with T. cruzi, and Chagas disease causes approximately 45,000 deaths annually. In the past, Chagas disease was a rarity in the United States. However, large scale immigration to the United States from Central America has changed the situation dramatically. An estimated 50,000 to 100,000 T. cruzi infected immigrants currently reside in the United States. Immunosuppression of patients with T. cruzi infection can result in reactivation of acute infection. Thus transplant patients and HIV-infected patients are at risk for recrudescence of acute Chagas disease.
The triatomine insects become infected by sucking blood from animals or humans with circulating trypomastigotes. The parasites multiply in the insects’ mid gut as epimastigotes. In the hind gut they transform into metacyclic forms of T. cruzi which are the infective form and then deposited in feces as the insect trypomastigote feeds. The trypanosomes penetrate through the bite wound or mucous membranes. T. cruzi is capable of penetrating into mammalian cells, where it develops into a mastigote form. Here the organism multiplies and forms a pseudocyst that ruptures with release of protozoan organisms that enter the bloodstream or invade adjacent cells. The cycle is completed when triatomine insects ingest human blood containing the parasites. Other modes for transmission of T. cruzi include blood transfusion in endemic areas, congenital infection, and laboratory accidents.
Programs in endemic countries have been recently implemented for vector and blood bank control. In particular, a major international control program involving Argentina, Bolivia, Brazil, Chile, Paraguay, and Uruguay initiated in 1991 has led to a marked decrease prevalence rate among the young and a prediction that if current trends continue, transmission will soon be virtually eliminated.
Clinical Presentation
The clinical course of T. cruzi presents as two distinct entities. Acute infection results with the initial encounter of the host and the organism. On the other hand, chronic Chagas disease involves late sequelae of the infection.
Acute Chagas disease usually occurs in children. While acute Chagas disease is usually mild, in immunocompromised patients the disease can be very severe. Only 1% of inhabitants of endemic areas who become infected develop clinically apparent acute disease. The most common site for the bite is the face. Urticaria often develops at the bite site, followed by an inflammatory nodule, the chagoma. The bite is often associated with Romana’s sign (the classic sign of acute Chagas disease), which is unilateral nonpurulent edema of the palpebral folds and an ipsilateral regional lymphadenopathy. After 2 or 3 weeks, parasitemia occurs with widespread dissemination, fever, lymphadenopathy, malaise, and peripheral edema. T. cruzi invades cells, resulting in the typical involvement of the heart, liver, gastrointestinal tract, and central nervous system. The cardiac disease which occurs in a small percentage is characterized by tachycardia, arrhythmia, hypotensions, cardiomegaly, and congestive heart failure. T. cruzi may invade the central nervous system and rarely causes meningoencephalitis. The mortality rate in the acute phase of Chagas disease is 10% to 20%, with most deaths secondary to cardiac disease or encephalitis. Generally acute Chagas disease resolves spontaneously over a 4- to 8-week period.
Chronic Chagas disease generally occurs in patients with no history of acute disease. It has a slow and insidious onset, occurring years and decades following the acute infection. Approximately 10% to 30% of individuals who harbor T. cruzi for years to decades develop symptoms of chronic Chagas disease. Cardiac involvement occurs in 20% to 40% of patients and presents as progressive cardiac enlargement in association with congestive heart failure and electrocardiographic evidence of arrhythmias. Emboli to the brain or other areas are a frequent complication. Involvement of chronic gastrointestinal disease is characterized by progressive development of megaesophagus or megacolon. Mortality rates of nearly 20% have been reported in association with chronic Chagas disease. Immunosuppression of persons harboring T. cruzi chronically may lead to reactivation of infection, often with greater severity than is associated with acute infection in immunocompetent individuals.
Diagnosis
The initial step in diagnosing acute Chagas disease is to determine if the patient’s history is consistent with exposure to T. cruzi. Risk factors include residence in an endemic area and birth to an infected mother. Definitive diagnosis in the acute stage of Chagas disease is made by demonstrating the protozoan T. cruzi on thin or thick smears of the blood or buffy coat. In immunocompromised patients other specimens (e.g., bone marrow, lymph node aspirates, skin lesion biopsies, endomyocardial tissue, cerebrospinal fluid, and pericardial fluid) should be examined microscopically.
Chronic Chagas disease is suggested by the characteristic clinical picture and confirmed by serologic tests and/or by biopsy demonstration of T. cruzi in tissue. Indirect immunofluorescence, compliment fixation, indirect hemaglutination and ELISA tests are available to detect anti T. cruzi IgG antibody. These serologic tests are associated with false-positive results. Because of this lack of specificity it has been suggested that specimens be tested with two or three serologic tests before being accepted as positive. In the United States, several kits for detecting antibodies to T. cruzi have been approved by the FDA. These include (i) Chagas EIA (Abbott Laboratories; Abbott Park, IL); (ii) Chagas IgG ELISA (Gull Laboratories; Salt Lake City, UT); and (iii) Chagas kit EIA (Hemagen Diagnostics; Waltham, MA). Recently, an ELISA based on recombinant antigen (Chaga test ELISA Recombinante v 3.0, Wierner Laboratories; Rosario, Argentina) was approved by the FDA.
Effects in Pregnancy
Chagas infection in pregnant women may cause spontaneous abortion, preterm birth, intrauterine growth restriction and stillbirth. In South America, 1% to 10% of spontaneous abortions are attributed to Chagas disease. Infection of the placenta usually can be demonstrated in these cases. T. cruzi reaches the placenta hematogenously and traverses the villi to the trophoblasts where following differentiation into the amastigote form, the organism remains in Hofbauer cells until it is released into the fetal circulation.
Congenital infection with Chagas disease is reported to occur in 1% to 4% of deliveries in women with serologic evidence of Chagas disease. The congenitally infected neonates tend to be preterm and/or small for gestational age. Approximately one third of congenitally infected infants die prior to 4 months of life. In addition, T. cruzi may be found in breast milk.
Treatment
Treatment of Chagas disease has been less than optimum. Currently, two drugs are used for treatment of
T. cruzi: a nitrofuran derivative, nifurtimox (Lampit, Bayer) and benznidazole (Radimil, Roche), a nitroimidazole derivative
(Table 22.8). Both drugs are available from the Centers for Disease Control, Drug Service (770-639-3670 weekdays and 770-639-2888 after hours). In pregnant women with evidence of cardiac or CNS disease, treatment should be started promptly. These agents reduce the duration and severity of acute and congenital Chagas disease. However, the parasite is eradicated from only 70% of treated patients. In the past it was debated as to whether effective treatment was available for chronic Chagas disease and whether chronic disease should be treated with nifurtimox or benzidazole. An international panel of experts recently recommended that all
T. cruzi-infected patients be treated with one of these agents regardless of clinical status or time since acute infection.