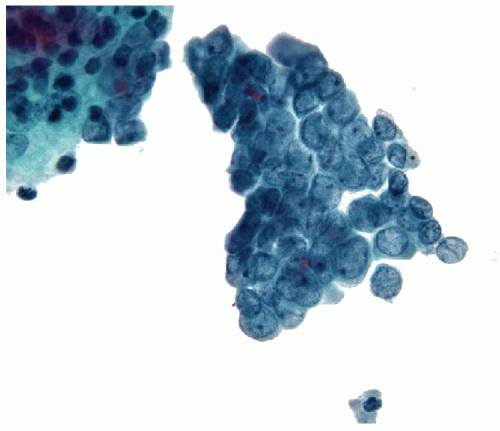
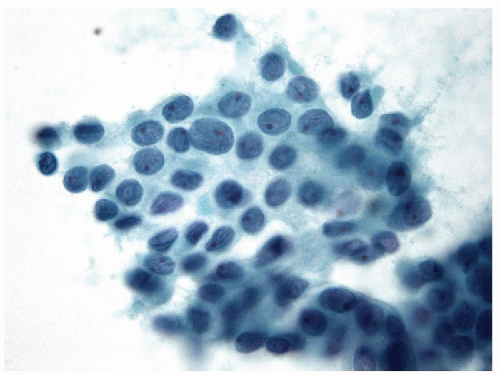
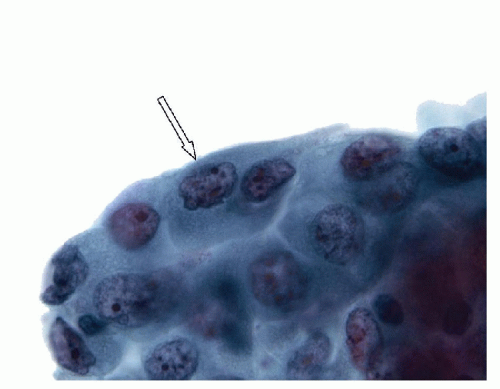
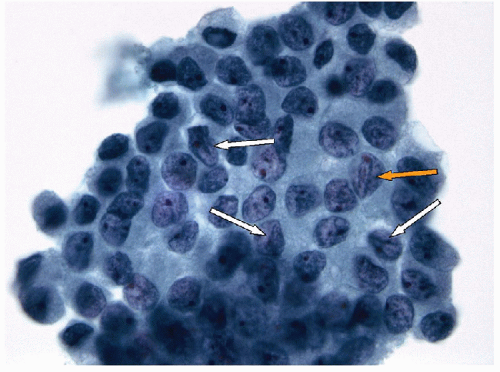
Papillary Lesions
GRAVES’ DISEASE
It is rare that a patient with Graves’ disease will undergo biopsy of the thyroid except in the event of a cold nodule developing within the hyperplastic gland. However, in that setting, it is possible that nonnodular tissue is included in the specimen, and it is important to distinguish the papillary hyperplasia from the pathology of the nodule in question. Surgery is an option for patients with this disorder, and total thyroidectomy provides an opportunity for the surgical pathologist to understand the morphology of this entity.
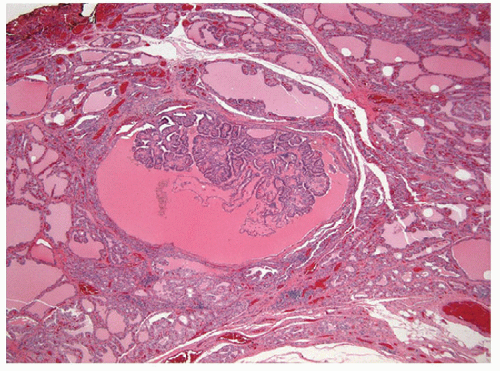
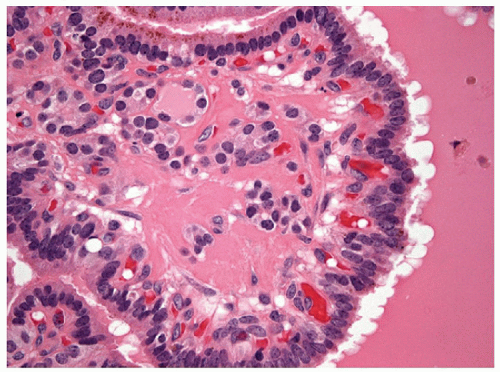
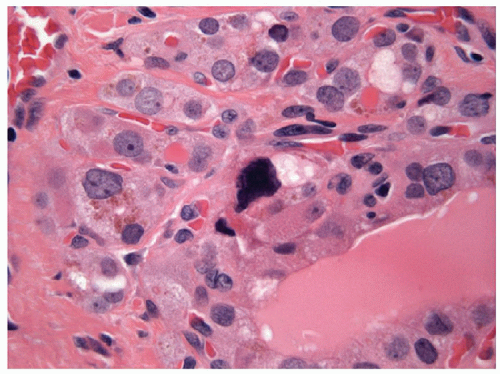
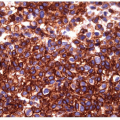
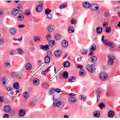
The pathology of Graves’ disease varies with the clinical status and therapy. In its florid form, the gland develops a diffuse increase in cellularity with epithelial hyperplasia forming papillary invaginations into the lumen of follicles that are devoid of colloid or contain only scant colloid with peripheral scalloping (Figs. 9.1 and 9.2, e-Fig. 9.1). The papillae have fibrovascular cores but usually are lined by tall columnar cells with basally oriented nuclei that are crowded but round and regular (Fig. 9.3, e-Fig. 9.2), without the irregularity of contour or the clearing with peripheral margination of chromatin that are the hallmarks of papillary carcinoma. Inflammatory cells are scarce and usually are not found in biopsies but rather are only found scattered in foci of the gland at surgical resection (Fig. 9.4).
The features of this disorder resemble papillary carcinoma superficially, since there is complex papillary architecture that in biopsies can mimic malignancy. In larger specimens, it is evident that the papillae in Graves’ disease are organized within follicles rather than having the haphazard pattern of papillary carcinoma. More critically, the cytologic features are not those of papillary carcinoma; the nuclei are crowded but usually retain their basal orientation. They do not have the convoluted membrane with grooves and inclusions that characterize papillary carcinoma, and they are usually evenly dark and basophilic rather than clear. The differential diagnosis based on these features is the hyperplastic or adenomatous nodule with papillary architecture discussed in the next section. In fine needle aspiration (FNA) specimens, marginal vacuoles or fireflare appearance in May-Grünwald-Giemsa stained smears was initially described as a distinctive feature of thyrotoxic goiter in hyperthyroidism. These vacuoles are thought to represent dilated endoplasmic reticulum, a manifestation of active pinocytosis containing colloid, or diffusion of thyroid hormone [1]. However, marginal vacuoles are found in various
nontoxic thyroid lesions [2], including papillary carcinoma and are therefore of little diagnostic assistance.
nontoxic thyroid lesions [2], including papillary carcinoma and are therefore of little diagnostic assistance.
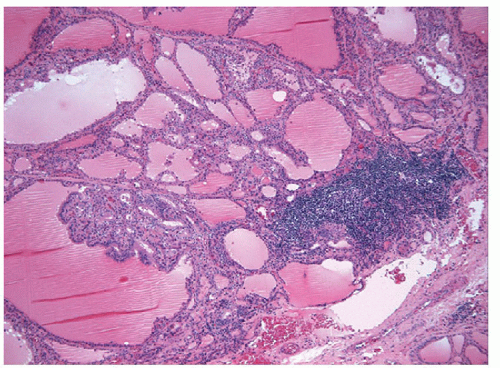
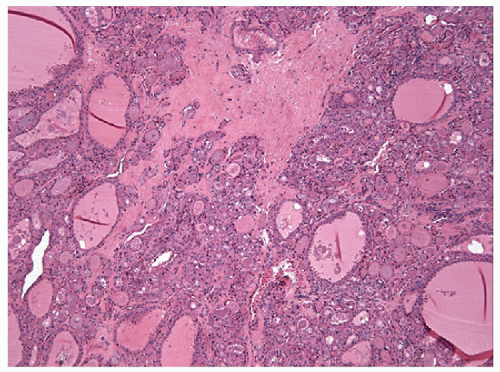
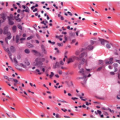
With the administration of antithyroid mediations (Tapazole, propylthiouracil), the hyperplasia recedes, follicles resume their more typical appearance, and colloid accumulates within their lumens (Fig. 9.5, e-Fig. 9.3). Radioactive iodine administration results in additional changes, including fibrosis, moderate focal chronic inflammation, and the presence of conspicuous “smudged” nuclei and nuclei that are large, hyperchromatic, and cytologically atypical (Fig. 9.6, e-Fig. 9.4).
HYPERPLASTIC NODULES AND ADENOMAS WITH PAPILLARY ARCHITECTURE
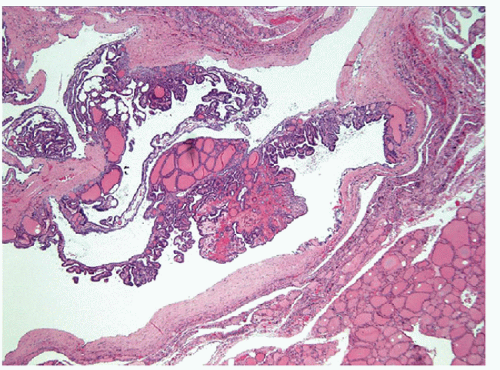
The “papillary hyperplastic nodule” of the thyroid is usually identified in girls, often in teenagers, in and around the age of menarche. These lesions present as solitary nodules, and they may be associated with clinical or subclinical hyperfunction; they are usually hot on radioiodine scan. These lesions are characterized by a unique architecture that is best recognized at low magnification. Although they do have true papillae with fibrovascular cores, the papillae are usually organized and have a centripedal pattern
within enlarged, distorted follicles. These nodules are distinguished from papillary carcinoma in that they are totally encapsulated or at least very well delineated without evidence of invasion, they often have central cystic change, they usually have at least focal subfollicle formation in the centers of broad edematous papillae, and most importantly, they do not have the nuclear features of papillary carcinoma (Figs. 9.7, 9.8, 9.9, 9.10, 9.11). Although one analysis of clonality has suggested that these are polyclonal hyperplasias [4], the detection of GNAS (also known as Gsα, gsp) or TSH receptor activating mutations in such nodules suggests that they are neoplasms [5, 6, 7, 8, 9]. These specific mutations that activate TSH signaling account for their functional hyperactivity as well as their structural morphology that resembles localized Graves’ disease. Their behavior is almost always benign. Some have advocated the name “papillary adenoma” for these tumors; while scientifically appropriate, this term carries historical connotations that some feel are unacceptable [10].
within enlarged, distorted follicles. These nodules are distinguished from papillary carcinoma in that they are totally encapsulated or at least very well delineated without evidence of invasion, they often have central cystic change, they usually have at least focal subfollicle formation in the centers of broad edematous papillae, and most importantly, they do not have the nuclear features of papillary carcinoma (Figs. 9.7, 9.8, 9.9, 9.10, 9.11). Although one analysis of clonality has suggested that these are polyclonal hyperplasias [4], the detection of GNAS (also known as Gsα, gsp) or TSH receptor activating mutations in such nodules suggests that they are neoplasms [5, 6, 7, 8, 9]. These specific mutations that activate TSH signaling account for their functional hyperactivity as well as their structural morphology that resembles localized Graves’ disease. Their behavior is almost always benign. Some have advocated the name “papillary adenoma” for these tumors; while scientifically appropriate, this term carries historical connotations that some feel are unacceptable [10].
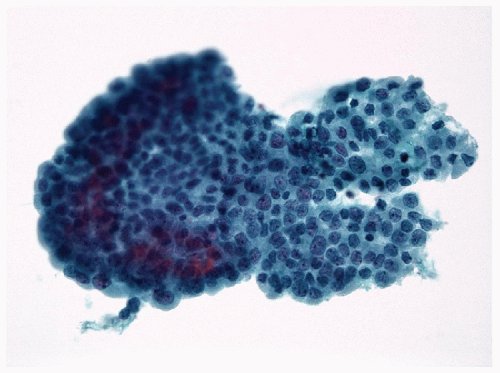
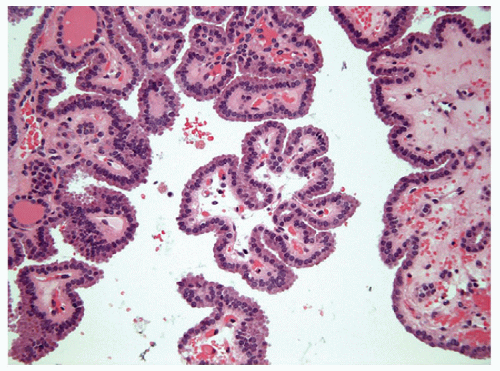 FIGURE 9.8 Papillary Hyperplastic Nodule. The papillae of papillary hyperplastic nodule contain true fibrovascular stalks that are apparent histologically and cytologically (Fig. 9.10) (hematoxylin and eosin stain). |
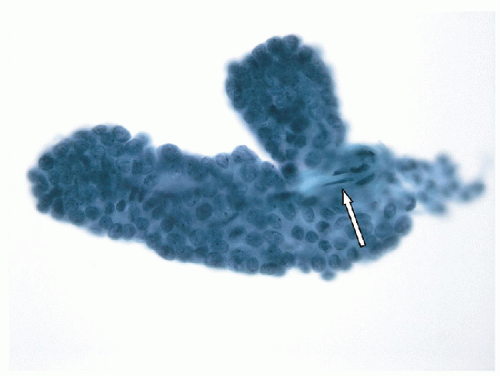
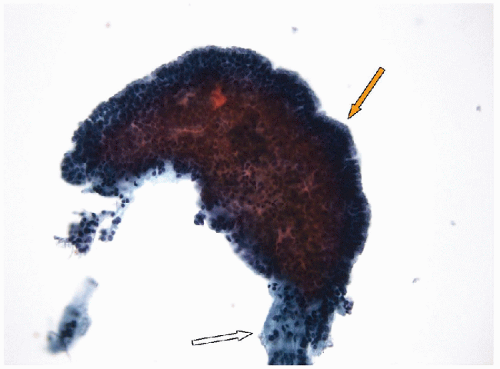 FIGURE 9.10 Papillary Hyperplastic Nodule. Aspiration of a papillary hyperplastic nodule may yield intact papilla as in this illustration where the fibrovascular stalk (white arrow) is visible and seen protruding from the still largely intact epithelial covering (orange arrow). These papillary stalks may generate a suspicion of papillary carcinoma, but it is critical to note that the epithelium lacks the nuclear features of papillary carcinoma (Fig. 9.11) (ThinPrep, Papanicolaou stain). |
In adults, one can have a similar histologic appearance in a “hot” nodule, that is, a thyroid nodule which is associated with clinical toxicity or subclinical hyperthyroidism and iodine uptake on scan. These lesions may be solitary, but in adults, they are more often seen in the setting of sporadic nodular goiter.
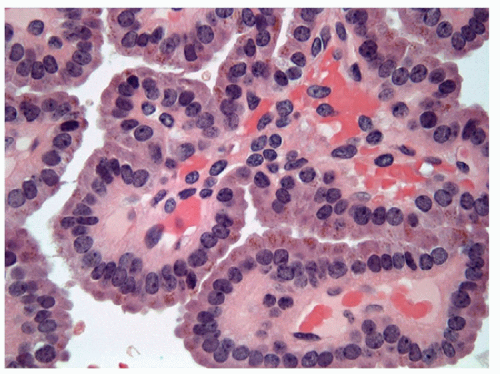
On fine needle aspiration and on histologic evaluation, particularly at frozen section, papillary hyperplastic nodules or adenomas can be very alarming and lead to a false-positive diagnosis of papillary carcinoma. Indeed, these entities give rise to well-formed papillae, but on higher magnification, they lack the cytologic criteria for the diagnosis of papillary carcinoma, including powdery nuclear chromatin, multiple micro- and/or macronucleoli, intranuclear cytoplasmic inclusions, and linear chromatin grooves [11, 12, 13]. Immunohistochemical studies have shown that HBME-1 can be useful to distinguish these lesions in histologic samples, since papillary carcinoma is frequently positive, but benign lesion are negative for this marker [14]. Molecular analysis GNAS or TSH receptor-activating mutations may be used but is usually not required.
PAPILLARY CARCINOMA, CLASSICAL TYPE, AND PAPILLARY VARIANTS
Papillary carcinoma represents the most common thyroid epithelial malignancy diagnosed in regions of the world where goiters are not endemic. Although malignant, these lesions are usually indolent and most have an excellent prognosis with a 20-year survival rate of 90% or better [15, 16]. They are often infiltrative with local invasion and even extrathyroidal extension. When these lesions do metastasize, they most often do so initially via lymphatics with initial regional lymph node metastases. Metastases beyond the neck are unusual in common papillary carcinoma.
Papillary carcinomas may be multifocal. This has been interpreted as reflective of intraglandular lymphatic dissemination. However, the identification of microcarcinomas in up to 24% of the population [17] and the detection of different clonal rearrangements [18] or X-chromosome inactivation patterns [19] in the different lesions from a single patient support the interpretation of multifocal primary lesions in most patients.
The terminology applied to this entity has been confused and confusing. Initially, papillary carcinoma was described as lesion with papillary architecture (e-Fig. 9.5). These lesions were known to also have follicular (e-Fig. 9.6) and mixed papillary and follicular patterns [10, 20, 21, 22, 23, 24, 25, 26]. With the recognition that papillary carcinoma is characterized by what the WHO has described as “a distinctive set of nuclear characteristics” [27],
the histologic diagnosis of papillary carcinoma of the thyroid became a cytologic diagnosis based on these nuclear features. While this recognition has enhanced the utility of FNA in the diagnosis of thyroid nodules, it has also led to controversy about the classification of papillary carcinomas with pure follicular architecture [28, 29].
the histologic diagnosis of papillary carcinoma of the thyroid became a cytologic diagnosis based on these nuclear features. While this recognition has enhanced the utility of FNA in the diagnosis of thyroid nodules, it has also led to controversy about the classification of papillary carcinomas with pure follicular architecture [28, 29].
Molecular studies have expanded the understanding of this controversy. Exclusive, nonoverlapping activating genetic alterations involving BRAF, RET, and NTRK are found in the majority of cases of papillary carcinoma with papillary architecture (Figs. 9.12, 9.13, 9.14, 9.15, 9.16) including multiple variants discussed in next sections with two exceptions [30, 31]. The activating BRAFV600E mutation is the most common, followed by other genetic alterations, including rearrangements such as RET/PTC or involving TRK; these yield a gene expression profile that has been described as “BRAF like” [30]. Rare reports of mutations in STK11, the gene responsible for Peutz-Jeghers syndrome, may also be implicated in the pathogenesis of papillary carcinoma [32], but this was associated with a BRAFV600E mutation as well. In contrast, tumors with nuclear atypia and pure follicular architecture harbor RAS mutations and other genetic alterations that are also found in follicular adenomas and carcinomas [30]. Thus, tumors classified as “follicular variant papillary carcinoma” are more akin to follicular neoplasms genetically. The impact of nuclear morphology in this field should be reconsidered for follicular lesions [33], and this subject will be covered in Chapter 10.
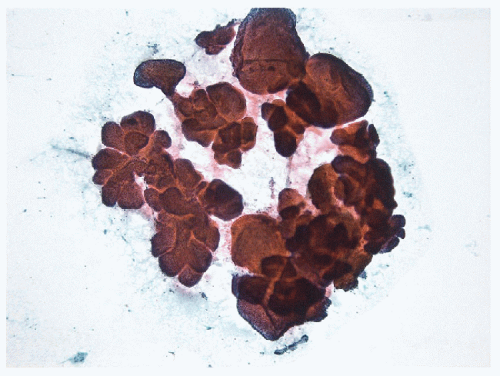 FIGURE 9.12 Papillary Carcinoma. Papillae from a conventional papillary carcinoma may present as complex three-dimensional structures when aspirated intact (direct smear, Papanicolaou stain). |
BRAF, a proto-oncogene on 7q24, encodes a serine/threonine kinase that transduces regulatory signals through the RAS/RAF/MEK/ERK cascade. Gain-of-function BRAF mutation results in aberrant activation of ERK signaling implicated in tumorigenesis of several human cancers such
as melanoma and colon carcinoma [34]. Several point mutations of exon 15 have been identified in thyroid cancers; BRAFV600E is the most common in sporadic papillary thyroid carcinoma [34, 35, 36] and is characteristic of papillary carcinomas with papillary architecture. This BRAF mutation occurs in papillary microcarcinomas and is considered to be an early event in thyroid follicular cell transformation. Although some studies suggested that BRAF is a predictor of aggressive behavior [37], more recent data suggest that the information obtained by these molecular studies actually reflected segregation of papillary from follicular variant papillary carcinomas; in pure groups of papillary lesions, BRAF mutation does not add further prognostic information alone [30, 38], and it has become clear that papillary lesions are more aggressive than follicular variant papillary carcinoma [39]. As discussed under “Prognostic features of papillary carcinoma,” (p. 155) additional molecular events are required to support more aggressive behavior [30, 40, 41]. The clinical application of BRAF inhibitors that would provide predictive value for patients with advanced disease who may benefit from this therapy remains to be proved [42, 43, 44, 45].
as melanoma and colon carcinoma [34]. Several point mutations of exon 15 have been identified in thyroid cancers; BRAFV600E is the most common in sporadic papillary thyroid carcinoma [34, 35, 36] and is characteristic of papillary carcinomas with papillary architecture. This BRAF mutation occurs in papillary microcarcinomas and is considered to be an early event in thyroid follicular cell transformation. Although some studies suggested that BRAF is a predictor of aggressive behavior [37], more recent data suggest that the information obtained by these molecular studies actually reflected segregation of papillary from follicular variant papillary carcinomas; in pure groups of papillary lesions, BRAF mutation does not add further prognostic information alone [30, 38], and it has become clear that papillary lesions are more aggressive than follicular variant papillary carcinoma [39]. As discussed under “Prognostic features of papillary carcinoma,” (p. 155) additional molecular events are required to support more aggressive behavior [30, 40, 41]. The clinical application of BRAF inhibitors that would provide predictive value for patients with advanced disease who may benefit from this therapy remains to be proved [42, 43, 44, 45].
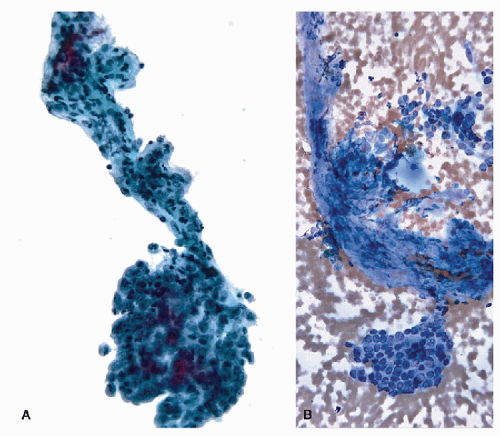
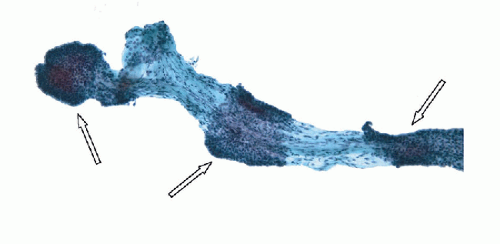 FIGURE 9.14 Papillary Carcinoma. Denudation of the epithelium from papillae (degloving injury to the papillae) is a frequent occurrence and can be accentuated during resuspension in ThinPrep slide production. This image shows partial denudation of a fibrovascular stalk of a papillary carcinoma where a few fragments of epithelium are preserved (arrows). The degloving injury generates separate epithelial fragments and naked fibrovascular stalks (Figs. 9.15 and 9.16) (ThinPrep, Papanicolaou stain). |
RET was the first activated receptor tyrosine kinase identified in thyroid cancer. The proto-oncogene on 10q11.2 encodes a transmembrane receptor tyrosine kinase with four cadherin-related motifs in the extracellular domain [34]. RET is normally expressed in the developing central and peripheral nervous systems and is essential for renal organogenesis and enteric neurogenesis. Glial-derived neurotrophic factor (GDNF) ligands and GDNF family receptor-α (GFRα) bind the extracellular domain of
RET to form a trimeric complex that induces tyrosine kinase autophosphorylation and activates several signaling pathways including ERK, PI3K, p38, and JUNK. Gain-of-function mutations of RET are involved in sporadic and familial C-cell-derived medullary thyroid carcinoma including multiple endocrine neoplasia (MEN) 2A, MEN 2B, and familial medullary thyroid carcinoma [46, 47, 48]. In contrast, more than 15 chimeric oncogenes, designated RET/PTC, are implicated in the development of papillary thyroid carcinoma [49]. Somatic chromosomal rearrangement leads to fusion of the 3′-terminal sequence of RET encoding the tyrosine kinase domain and 5′-terminal sequences of heterologous genes [49, 50, 51]. Although wildtype RET is not normally expressed in follicular cells, RET/PTC chimeric oncoproteins lacking a signal peptide and transmembrane domain are expressed in the cytoplasm of follicular cells under the control of the newly acquired promoters. The constitutive activation of the tyrosine domain in
the carboxyl-terminal end of RET/PTC induces signaling pathways within thyrocytes and causes cellular transformation in transgenic mice [52, 53, 54]. The high frequency of RET rearrangements in subclinical papillary thyroid microcarcinomas is consistent with these changes representing early events in the neoplastic process [18]. RET/PTC has been implicated in the development of the characteristic nuclear features of papillary carcinoma through alteration of the nuclear envelope and chromatin structure [55]. Heterogeneity of RET rearrangements in a single tumor has been identified and was interpreted as indicative of a relatively late event; however, an alternative interpretation is that multiple and distinct rearrangements signify multifocal transforming events in benign clonal neoplasms [56]. RET/PTC rearrangements have also been reported in thyroids with Hashimoto’s thyroiditis [57], and this has been the major criticism hampering the application of this molecular marker to biopsy diagnosis; however, these findings are controversial and may reflect either PCR contamination or the presence of common microscopic nodules of papillary thyroid carcinoma [17], since many studies that have excluded these possibilities do not confirm the data [18, 58].
RET to form a trimeric complex that induces tyrosine kinase autophosphorylation and activates several signaling pathways including ERK, PI3K, p38, and JUNK. Gain-of-function mutations of RET are involved in sporadic and familial C-cell-derived medullary thyroid carcinoma including multiple endocrine neoplasia (MEN) 2A, MEN 2B, and familial medullary thyroid carcinoma [46, 47, 48]. In contrast, more than 15 chimeric oncogenes, designated RET/PTC, are implicated in the development of papillary thyroid carcinoma [49]. Somatic chromosomal rearrangement leads to fusion of the 3′-terminal sequence of RET encoding the tyrosine kinase domain and 5′-terminal sequences of heterologous genes [49, 50, 51]. Although wildtype RET is not normally expressed in follicular cells, RET/PTC chimeric oncoproteins lacking a signal peptide and transmembrane domain are expressed in the cytoplasm of follicular cells under the control of the newly acquired promoters. The constitutive activation of the tyrosine domain in
the carboxyl-terminal end of RET/PTC induces signaling pathways within thyrocytes and causes cellular transformation in transgenic mice [52, 53, 54]. The high frequency of RET rearrangements in subclinical papillary thyroid microcarcinomas is consistent with these changes representing early events in the neoplastic process [18]. RET/PTC has been implicated in the development of the characteristic nuclear features of papillary carcinoma through alteration of the nuclear envelope and chromatin structure [55]. Heterogeneity of RET rearrangements in a single tumor has been identified and was interpreted as indicative of a relatively late event; however, an alternative interpretation is that multiple and distinct rearrangements signify multifocal transforming events in benign clonal neoplasms [56]. RET/PTC rearrangements have also been reported in thyroids with Hashimoto’s thyroiditis [57], and this has been the major criticism hampering the application of this molecular marker to biopsy diagnosis; however, these findings are controversial and may reflect either PCR contamination or the presence of common microscopic nodules of papillary thyroid carcinoma [17], since many studies that have excluded these possibilities do not confirm the data [18, 58].
There is wide variation in the reported frequency of RET/PTC rearrangements in papillary thyroid carcinomas, related to geographic location, radiation exposure, and detection methods [59]. The high incidence of RET rearrangements in childhood papillary thyroid carcinomas following the Chernobyl accident suggests a role for radiation damage in the genesis of these paracentric inversions [60, 61]. Analysis of chromatin patterns in thyroid follicular cells identified physical proximity of the partners involved in the illegitimate recombination of these rearrangements [62], providing a plausible scenario for radiation-related susceptibility. In nonradiation induced sporadic papillary carcinomas, RET/PTC-1 and RET/PTC-3 are the most common types of RET rearrangements, identified in approximately 40% and 15% of cases, respectively [59, 63, 64]. These fusions represent the second most frequent genomic alterations in classical papillary thyroid carcinomas [30]; however, the incidence of these rearrangements has been decreasing over time [65].
The receptor tyrosine kinase NTRK1 was the second identified subject of chromosomal rearrangement in thyroid tumorigenesis. The NTRK1 (TRK, TRKA) proto-oncogene on 1q22 encodes the transmembrane tyrosine kinase receptor for nerve growth factor. NTRK1 is typically restricted to neurons of the sensory spinal and cranial ganglia of neural crest origin and regulates neuronal growth and survival. NTRK1 rearrangements, showing ectopic expression and constitutive activation of the tyrosine kinase analogous to RET rearrangements, have been noted in 5% to 13% of sporadic but in only 3% of post-Chernobyl childhood papillary thyroid carcinomas [34]. To date, TPM3, TPR, and TFG have been identified as fusion partners forming chimeric oncogenes designated as TRK, TRK-T1 and T2, TRK-T3, respectively. The prevalence of each fusion type is nearly equal in sporadic papillary thyroid carcinomas, while TPM3-NTRK is more
frequent than other NTRK1 rearrangements in post-Chernobyl childhood papillary thyroid carcinomas. The relatively lower incidence of these rearrangements makes them less valuable as routine markers for diagnostic biopsies [30].
frequent than other NTRK1 rearrangements in post-Chernobyl childhood papillary thyroid carcinomas. The relatively lower incidence of these rearrangements makes them less valuable as routine markers for diagnostic biopsies [30].
Nuclear Features of Papillary Carcinoma
While the presence of papillary structures is not pathognomonic of papillary carcinoma, the identification of a lesion with a papillary architecture should prompt a careful search for the nuclear features of papillary carcinoma. Some of the changes seen in papillary carcinoma are part of the neoplastic process and less specific to papillary carcinoma itself. Common to most thyroid neoplasia is the presence of nuclear enlargement, loss of nuclear roundness, increased chromatin granularity, and increased prominence of the nucleolus.
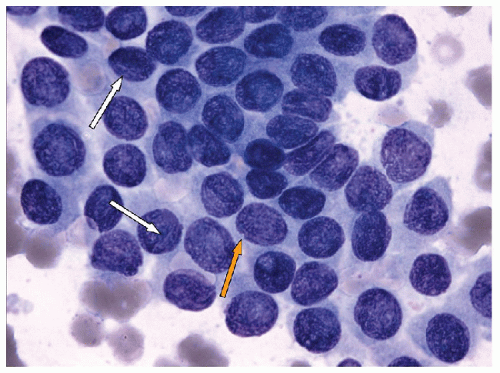
NUCLEAR ENLARGEMENT AND ELONGATION. The nuclei in thyroid neoplasia enlarge and do so out of proportion to any increase in cytoplasm, resulting in an increased nuclear/cytoplasmic (N/C) ratio. This nuclear enlargement may also be recognized by any of the following three manifestations of increased N/C ratio (Figs. 9.17 and 9.18):
1. Loss of the apparent basal polarization of the nuclei (seen histologically)
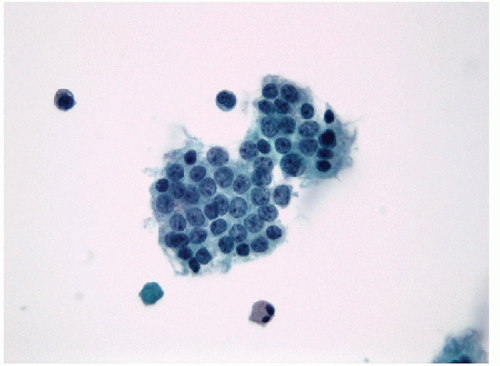
2. Nuclear crowding and overlapping in the epithelial fragments (seen cytologically and histologically)
3. Transition from monolayered sheets to syncytial aggregates (seen in cytological preparations)
The nuclear enlargement is often inconsistent from cell to cell, generating a mild degree of pleomorphism, and the nuclear enlargement is asymmetrical, resulting in a nucleus that has lost its typical spherical shape and becomes ovoid (Fig. 9.19). Hypothetically, this reflects the disordered nuclear scaffolding that occurs as a result of the oncogenesis of papillary carcinoma and manifests a number of other profound alterations in nuclear structure that take on diagnostic importance.
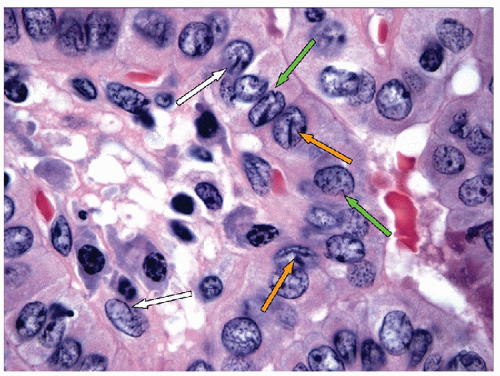
NUCLEAR MEMBRANE IRREGULARITY, NUCLEAR GROOVES, AND INTRANUCLEAR INCLUSIONS. The nuclear membranes of papillary carcinoma show irregularities of varying degrees. Most nuclei are oval with smooth, regular margins. However, it is not uncommon to find nuclei with strikingly irregular nuclear membranes, resulting in a “crumpled paper” or “raisinlike” appearance (Fig. 9.20). One manifestation of this nuclear membrane irregularity is the nuclear groove. The nuclear grooves seen in papillary carcinoma have been given a variety of names, including linear chromatin ridge, chromatin band, nuclear folds, nuclear crease, or more accurately invaginations. Nuclear grooves actually reflect an invagination of
the nuclear membrane that runs parallel to the long axis of the elongated nucleus [66, 67] (Figs. 9.21, 9.22, 9.23, 9.24, e-Figs. 9.7, 9.8, 9.9). Many grooves are fine and difficult to resolve by light microscopy. When well oriented and well resolved, one can appreciate that the groove is composed of two parallel lines representing the edges of the nuclear groove where the membrane has invaginated (Figs. 9.21 and 9.24, e-Figs. 9.7, 9.8, 9.9). If the orientation of the nucleus is such as to view the groove on edge, the invagination may be appreciated as a “notch” (Fig. 9.22).
the nuclear membrane that runs parallel to the long axis of the elongated nucleus [66, 67] (Figs. 9.21, 9.22, 9.23, 9.24, e-Figs. 9.7, 9.8, 9.9). Many grooves are fine and difficult to resolve by light microscopy. When well oriented and well resolved, one can appreciate that the groove is composed of two parallel lines representing the edges of the nuclear groove where the membrane has invaginated (Figs. 9.21 and 9.24, e-Figs. 9.7, 9.8, 9.9). If the orientation of the nucleus is such as to view the groove on edge, the invagination may be appreciated as a “notch” (Fig. 9.22).
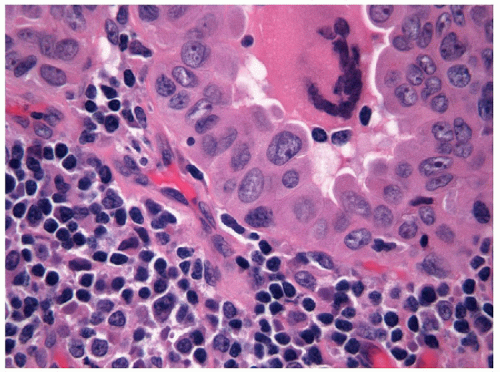
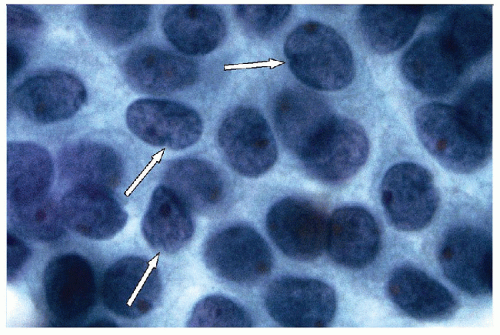 FIGURE 9.22 Nuclear Grooves. When nuclear grooves are viewed from the side, the profile of the invagination becomes apparent as a valley or “notch” (arrows) (ThinPrep, Papanicolaou stain). |
It must be noted that nuclear grooves in follicular epithelial cells are also seen in a variety of settings, most notably in lymphocytic thyroiditis and are not specific for papillary carcinoma. Quantification studies
have shown that papillary carcinoma tends to have more grooves than other lesions [68, 69, 70, 71, 72, 73, 74] but have not shown that a specific number of grooves establishes a definitive diagnosis. One further caveat is that nuclear grooves are of diagnostic help only when identified within a follicular epithelial cell. Macrophages (histiocytes) are characterized by elongated oval nuclei with nuclear grooves that can mimic papillary carcinoma [75], and clearly, the identification of grooves in histiocytes is of no diagnostic value with regards to papillary carcinoma.
have shown that papillary carcinoma tends to have more grooves than other lesions [68, 69, 70, 71, 72, 73, 74] but have not shown that a specific number of grooves establishes a definitive diagnosis. One further caveat is that nuclear grooves are of diagnostic help only when identified within a follicular epithelial cell. Macrophages (histiocytes) are characterized by elongated oval nuclei with nuclear grooves that can mimic papillary carcinoma [75], and clearly, the identification of grooves in histiocytes is of no diagnostic value with regards to papillary carcinoma.
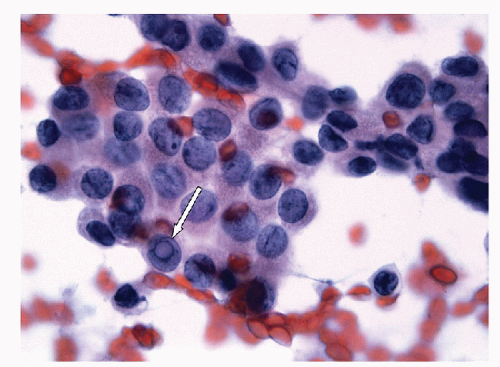
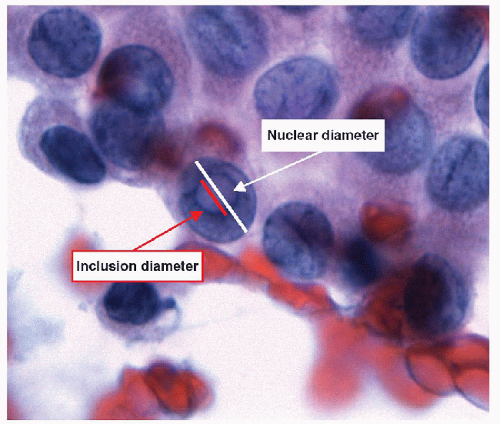
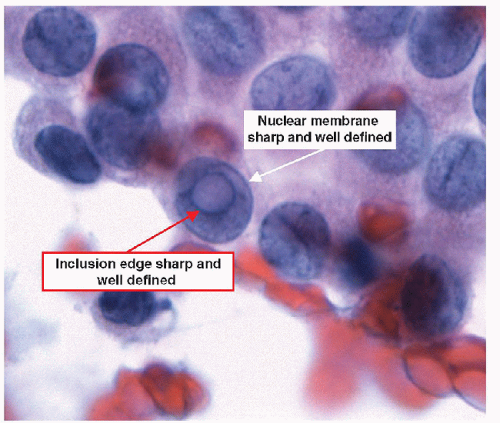
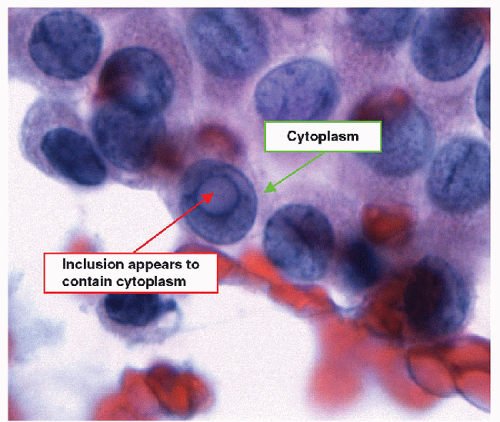
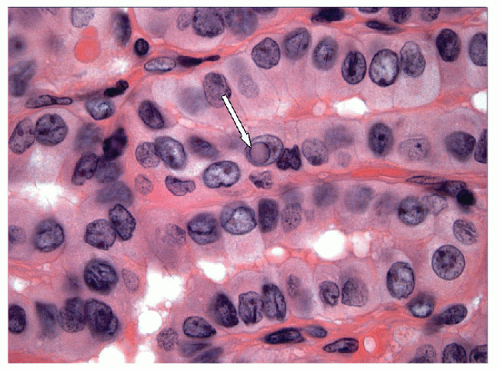
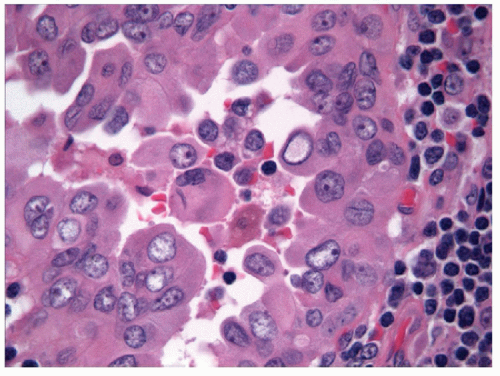
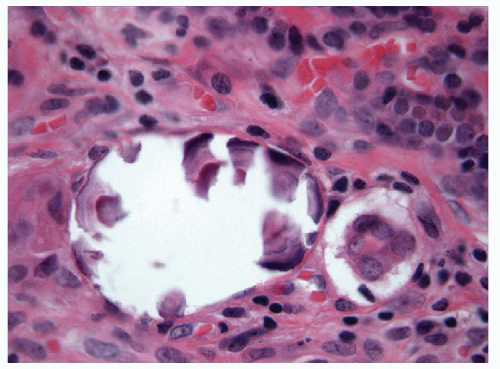
When carried to an extreme and reflecting profound nuclear skeletal derangement, invagination of the nuclear membrane produces intranuclear cytoplasmic pseudoinclusions [66, 68, 74, 76], shortened to “intranuclear inclusions.” Intranuclear inclusions have a high specificity for papillary carcinoma, although not 100%. However, to have this degree of specificity, the object in question must be an intranuclear inclusion and not a mimic such as an area of chromatin pallor. Thus to be considered an intranuclear inclusion, the object must fulfil four criteria. These criteria have been derived to achieve highly specific cytologic diagnoses but are equally applicable to histologic preparations (Figs. 9.25, 9.26, 9.27, 9.28, 9.29, e-Figs. 9.10, 9.11, 9.12, 9.13, 9.14, 9.15, 9.16).
1. Size criterion-the diameter of an intranuclear inclusion must be at least one-quarter the diameter of the nucleus (Fig. 9.26).
2. Edge criterion-the inclusion must have sharply defined borders and should be round and regular (Fig. 9.27).
3. Contents criterion-the inclusion should contain material that appears similar to the cytoplasm of the cell (Figs. 9.28 and 9.29).
4. Epithelial cell criterion-the cell housing the inclusion must be an identifiable follicular epithelial cell (Fig. 9.29).
The most overly rigid criterion is the size requirement. There are certainly many intranuclear inclusions that are smaller than this criterion allows. However, artifacts that mimic intranuclear inclusion tend to be small, and this size criterion helps eliminate the possibility of misinterpreting these mimics.
The edge of the intranuclear inclusion is generated by the invaginated nuclear membrane, and as such must be as sharply defined as the other portions of the nuclear membrane and the inclusion itself should appear round and regular. This criterion is also overly rigid, for it requires the nucleus to have an ideal orientation to the observer. Thus, a perfectly orientated intranuclear inclusion will have a very sharp, crisp edge. When the inclusion is off angle in cytologic preparations, the intranuclear inclusion is viewed through some of the nucleoplasm, and the edges may not be as clear and defined. In histologic preparations, tangential sectioning may rarely produce a similar problem. Relaxation of requirement for sharpedged inclusions results in a risk of considering areas of chromatin clearing as inclusions, and these regions of chromatin clearing do not convey the same diagnostic significance as the inclusion.
The third criterion for an intranuclear inclusion requires the identification of cytoplasm within the inclusion, and this is most often apparent in the tinctorial characteristics of the inclusion compared to the cytoplasm outside the nucleus. In Papanicolaou-stained cytologic preparations, the inclusion should be cyanophilic to faintly eosinophilic; in Romanowskystained preparations, the inclusion is pale blue; and in material stained with hematoxylin and eosin, the inclusion is pink. In histologic preparations, this criterion is absolute. However, in cytologic preparations, this criterion may again be considered somewhat harsh, as an inclusion oriented, so there is nucleoplasm between the observer and the inclusion will appear more basophilic due to the overlying chromatin.
The final criterion is not overly harsh and must be met. For an intranuclear inclusion to be accepted, it must be seen within a follicular epithelial cell. Intranuclear inclusions have no specificity for papillary carcinoma if they are not within follicular epithelial cells.
The presence of intranuclear inclusions is not pathognomonic of papillary carcinoma even when the four criteria above are satisfied. Intranuclear inclusions are known to occur in other lesions such as medullary carcinoma. They have also been reported in follicular epithelial cells in Hashimoto’s thyroiditis [77]. Some investigators have proposed that a specific number of inclusions can predict papillary carcinoma [68, 71, 78]; however, the frequency of intranuclear inclusions varies among the subtypes of papillary carcinoma. For example, the follicular variant of papillary carcinoma is notorious for having fewer intranuclear inclusions that
often do not appear to be as well developed as in conventional papillary carcinomas. For this reason, intranuclear inclusions are not a prerequisite for the diagnosis of papillary carcinoma, although in cytologic practice, the potential to misdiagnosis increases when intranuclear inclusions are lacking.
often do not appear to be as well developed as in conventional papillary carcinomas. For this reason, intranuclear inclusions are not a prerequisite for the diagnosis of papillary carcinoma, although in cytologic practice, the potential to misdiagnosis increases when intranuclear inclusions are lacking.
OPTICALLY CLEAR NUCLEI. The chromatin of neoplastic thyroid cells is altered and frequently becomes more pale and granular (powdery) in comparison to resting thyroid cells. Whereas chromatin changes are seen in many different thyroid neoplasms and are relatively nonspecific, the chromatin structure in papillary carcinoma is predisposed to the development of peripheral margination. As a result, the center of the nucleus develops a “ground-glass” appearance [79] or, when pronounced, appears to be optically clear. The chromatin is pushed to the edge of the nucleus, and the central clearing with thickened outline of the nucleus yield an appearance that resembles the large oval eyes of the cartoon character Orphan Annie, hence the term “Orphan Annie-eye nuclei” (Fig. 9.30, e-Fig. 9.17). This is a fixation artifact [80] seen in formalin-fixed tissue and is not typically evident in frozen sections. It should be noted that the occurrence of optically clear nuclei is variable and influenced by the fixation conditions and has been reported in lesions other than papillary carcinoma [80]. Optically clear nuclei are not described in routinely processed FNA of papillary carcinoma. However, it has been reported that optically clear nuclei can be
induced in direct smears of the FNA specimens if the smears are first airdried and then rehydrated followed by staining with a modified Pap stain [81, 82, 83].
induced in direct smears of the FNA specimens if the smears are first airdried and then rehydrated followed by staining with a modified Pap stain [81, 82, 83].
NUCLEOLI. The nucleoli of thyroid neoplasms are increased in prominence in comparison to resting follicular epithelium. The nucleoli do not take on the size and intensity of those seen in adenocarcinomas that arise elsewhere in the body but are instead seen as one to three micronucleoli, positioned toward the nuclear membrane. As usual, nucleolar feature is the development of “bare nucleoli” in which the chromatin surrounding the nucleolus is cleared, giving the appearance of the nucleolus residing within a hole (e-Fig. 9.18).
Other Features Seen in Papillary Carcinoma
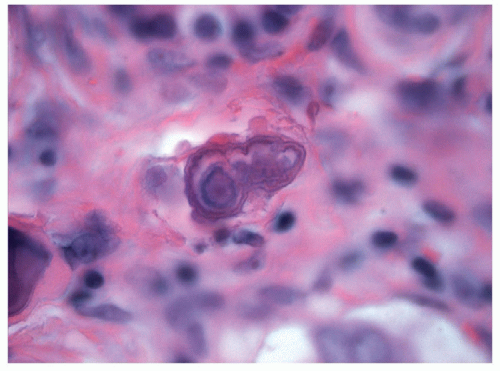
PSAMMOMA BODIES. Psammoma bodies are calcified, hematoxyphilic, concentrically laminated, spherical bodies (Figs. 9.31 and 9.32). It is common for psammoma bodies to fuse together, making larger concretions, and thereby lose their spherical form. Psammoma bodies are found in 40% to 50% of classical papillary carcinomas, either in the tumor or in the surrounding nontumorous thyroid. In contrast, they are distinctively uncommon in other variants of papillary carcinoma. Psammoma bodies alone are not diagnostic of papillary carcinoma. However, the presence of true psammoma bodies should be considered suspicious and prompt a careful search for a neoplasm with the nuclear changes of papillary carcinoma.
Dense colloid, amyloid, collagenous structures, foreign bodies, and dystrophic calcification may mimic the appearance of psammoma bodies. These mimics are usually distinguished by their lack of concentric laminations that characterize psammoma bodies.
Dense colloid, amyloid, collagenous structures, foreign bodies, and dystrophic calcification may mimic the appearance of psammoma bodies. These mimics are usually distinguished by their lack of concentric laminations that characterize psammoma bodies.
The pathogenesis of psammoma bodies is a matter of debate. Historically, it was felt that psammoma bodies resulted from either thickening of the papilla’s basal lamina followed by vascular thrombosis, calcification and tumor cell necrosis, or necrosis and calcification of intralymphatic tumor thrombi [84]. However, these theories do not account for the formation of psammoma bodies in nonpapillary tumors (such as meningioma) nor for the observations of intracytoplasmic psammoma bodies (Figs. 9.33, 9.34, 9.35). Thus, alterative theories have suggested calcification of hyaline stromal structures [85] or mechanisms similar to those proposed for serous tumors involving nanobacteria acting as a nidus for psammoma body formation [86, 87].
COLLOID. Colloid depletion is relatively common in papillary carcinoma. When present, the colloid of papillary carcinoma has some notable features. Histologically, the colloid is often darker staining than in the normal thyroid, and often there is peripheral scalloping of the colloid in follicles of irregular shape (e-Fig. 9.19). Cytologically, the so-called “chewing gum colloid” of papillary carcinoma appears more dense and “sticky” than usual (e-Figs. 9.20 and 9.21). In either case, these changes are not diagnostically specific.
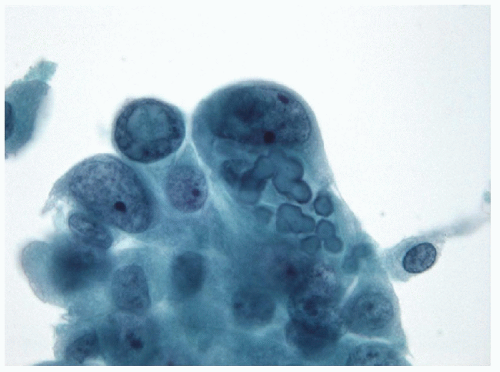 FIGURE 9.33 Psammoma Bodies. The pathogenesis of psammoma bodies is somewhat debated. In this example and the following figures (Figs. 9.34 and 9.35), it is apparent that psammoma bodies may have an intracytoplasmic origin that does not support the hypothesis of cellular necrosis leading to psammoma body development. In this image, epithelial cells of a papillary carcinoma are seen to contain intracytoplasmic psammoma bodies in which a central pinpoint nidus is evident with the first few layers of lamination (ThinPrep, Papanicolaou stain).
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|