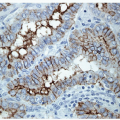
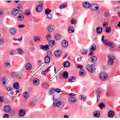
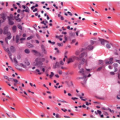
The Cytologic Approach to Diagnosis of Thyroid Pathology—Technical Aspects
FINE NEEDLE ASPIRATION OF THE THYROID
Fine needle aspiration (FNA) appears to be an exceedingly simple procedure. The target lesion is fixed in position with one hand. Using the other hand and often a syringe holder with syringe and attached needle, the needle is inserted through the skin into the lesion. Suction is applied by drawing back on the plunger, and the needle is moved back and forth within the lesion. After a number of these needle strokes, the suction is released, and the needle is withdrawn to distribute the collected sample for examination. Despite its apparent simplicity, one of the greatest problems in FNA of the thyroid is unsatisfactory samples [1, 2, 3]. The primary reasons for unsatisfactory sampling relate to the target itself, a vascular organ often with cystic changes and FNA technique that is counterproductive due to reliance on negative aspiration pressure, use of large bore needles, and failure to control hemostasis.
The vascular nature of the thyroid gland makes it difficult to obtain an adequate sampling as there is a tendency for hemodilution to occur during the procedure. Furthermore, many thyroid lesions are cystic, and this cyst fluid, which in itself is not of diagnostic value, has a tendency to flood the FNA sample rendering the entire sampling of little diagnostic value. The means to contend with the issue of hemodilution is adherence to careful FNA technique as suggested in the next section. The problem incurred due to cystic targets is best approached by utilization of ultrasound guidance to target the solid elements of cystic lesion.
One of the first steps to achieve better FNA samples is to abandon the myth that the aspiration generates the sample. FNA samples of thyroid can be successfully obtained using either aspiration or nonaspiration techniques [4, 5, 6, 7, 8, 9]. Aspiration does help to retain the collected sample, potentially generating a better yield, but aspiration also causes hemodilution leading to an unsatisfactory sample.
Physics dictates that with suction alone, the sample with the least resistance to flow will enter the needle. In FNA of a solid target, the material with the least resistance to flow is tissue fluid, blood, and possibly inflammatory cell populations if present. Even with inflammatory cell populations, collection through aspiration alone will meet with limited success unless the inflammation is in the form of an abscess, since even inflammatory cells have attachment, albeit weak, to the surrounding stroma. Aspiration of a cystic lesion will drain the cyst fluid and not collect any tissue. In fact, cyst fluid is pressurized in comparison to the surrounding tissue and thus immediately upon entry into a cyst, the needle and syringe becomes flooded with cyst fluid even without the application of negative pressure. Tissue is structured, and the tissue elements are anchored in place. The only way by which tissue can be collected during FNA is to disrupt the tissue structure, thereby liberating the component elements from their surroundings and allowing them to enter into the bore of the needle. The disruption of the tissue occurs as the sharpened beveled edge of the needle cuts through the tissue during the forward movement of the needle. A single thrust (or stroke) of the needle into the tissue dislodges a minute quantity of the tissue including both epithelial and stromal elements that is then forced into the bore of the needle. It is during the backward movement of the stroke that the suction comes into play, for a portion of the dislodged material would be lost from the needle if suction were not present upon the backward movement of the needle. The aspiration does not generate any sample. Actually, aspiration is counterproductive as the blood liberated by the disruption of the capillaries during the needle stroke will enter into the needle preferentially. Each thrust of the needle through the lesion, also referred to as a “needle stroke,” generates a minute quantity of dislodged tissue fragments that are forced into the bore of the needle. It should be noted that the ease at which tissue is liberated will influence the quantity of sample collected. FNA is successful because the vast majority of lesions that we sample are predominantly epithelial in nature, and these epithelial components are easily dislodged from their surroundings. In neoplastic epithelial lesions, the epithelial elements appear to be even more readily separated from their surroundings. However, stromal elements are much more difficult to dislodge, and this in part explains why stromal elements are less well represented in FNA specimens than one would see in equivalent tissue sections, for example, in core needle biopsies. Furthermore, lesions rich in connective tissue elements, such as sclerotic tumors, do not provide good FNA samples. The aspirator is often aware of this situation when it arises, because the lesion appears to grip the needle, making the strokes difficult to perform and feeling “gritty.”
As aspiration is not necessary in order to collect the sample for FNA, a very pure sample may be obtained by simply using a needle alone without attached syringe. One disadvantage of the needle-alone approach is that the sample collected may be so pure that the production of direct smears may be difficult due to the viscosity of the sample. However, the reason for
avoidance of the needle-alone approach for FNA of thyroid lesions is the frequency of cystic lesions and those containing abundant colloid. Once the needle is first inserted into the lesion, aspiration is applied to evacuate any cystic fluid contents. If no fluid contents are present, either aspiration may be maintained and the needle strokes initiated or the aspiration may be released and then the needle strokes performed.
avoidance of the needle-alone approach for FNA of thyroid lesions is the frequency of cystic lesions and those containing abundant colloid. Once the needle is first inserted into the lesion, aspiration is applied to evacuate any cystic fluid contents. If no fluid contents are present, either aspiration may be maintained and the needle strokes initiated or the aspiration may be released and then the needle strokes performed.
After multiple needle strokes, the collected material actually fills the needle bore and is forced into the hub of the needle where the sample becomes visible to the aspirator; this is referred to as the “flashback” of the sample. At this stage, the needle strokes should be terminated. If there is an immediate flashback, then the FNA has been traumatic as the aspiration bed is now flooded with blood, and the possibility of obtaining an adequate sample from that needle is very low. If this occurs, the aspiration should be stopped with pressure applied to the aspiration site in order to establish hemostasis. Hemostasis is important not only to prevent development of a hematoma but also to prevent contamination of the aspiration site with blood with the hope that immediate repeat aspiration will more likely be successful.
Although any gauge of needle could be used for FNA, the principle of minimizing bleeding dictates that the smallest possible needle gauge should be chosen. The optimal needle size for biopsy of a palpable lesion is 27G or 25G; the latter has the advantage of greater rigidity with less bending of the needle during the aspiration procedure. Another myth that must be dispelled is that a bigger needle is better, based perhaps on the idea that a fine gauge needle will be too small to collect tissue fragments. Although the needles are of very small caliber, they are sufficiently large to collect abundant material, and intact papillary structures can be recovered from papillary carcinoma with 27G or 25G needles. Such fine needles also receive high patient acceptance and reduce discomfort, if only because of the psychological impact of telling the patient that the needle being used is much smaller than the one used to collect a blood sample.
Lesion immobilization is also important for obtaining adequate FNA samples. If the lesion is able to move during the procedure, as a needle is advanced, the lesion moves away from the force so that the net movement of the needle within the lesion is reduced substantially. As the movement of the needle through the lesion is critical for obtaining the sample, if the lesion moves with the needle, there is no cutting action and the effect is to blockade sample acquisition.
Multiple needle strokes are required to fill the needle bore with sample, but multiple passes are likely to be required in order to obtain sufficient tissue for evaluation and to ensure representation of the lesion cytologically. A pass is the complete process of collecting one sample with the use of one needle and multiple strokes. Each pass is repetition of the aspiration with a new needle, hopefully positioned within the lesion, but somewhat away for the site of first aspiration. The repositioning is intended to avoid the bloody field elicited by the first pass and to ensure that different portions of the
lesion are sampled. Some lesions are notorious for appearing deceptively bland and benign in one focus and then definitively diagnostic in another focus. The exact number of passes required to ensure representation of the lesion is a matter of debate and will be influenced by the experience of the aspirator, the quality of sample recovered, the size and nature of the lesion and the availability of immediate evaluation of sample quality or assessment or adequacy. Using very broad generalization, two or three passes will likely generate an adequate and representative sample under most circumstances, but five to six passes may be necessary for some lesions [10, 11, 12, 13].
lesion are sampled. Some lesions are notorious for appearing deceptively bland and benign in one focus and then definitively diagnostic in another focus. The exact number of passes required to ensure representation of the lesion is a matter of debate and will be influenced by the experience of the aspirator, the quality of sample recovered, the size and nature of the lesion and the availability of immediate evaluation of sample quality or assessment or adequacy. Using very broad generalization, two or three passes will likely generate an adequate and representative sample under most circumstances, but five to six passes may be necessary for some lesions [10, 11, 12, 13].
There is no question that ultrasound-guided FNA of the thyroid has allowed better visualization and needle placement into increasing smaller thyroid lesions and surrounding structures [14, 15, 16, 17]. Furthermore, the lesional characteristics on ultrasound may aid in target selection. There is actually little difference in the overall technique whether there is ultrasound visualization or palpation. One important technical consideration with ultrasound-guided FNA of the thyroid is to avoid contamination of the sample with ultrasound gel. The gel itself partially obstructs the needle bore, reducing the volume of sample collected, and the presence of gel in the sample can significantly hinder some types of liquid-based cytologic processing techniques. Thus, prior to insertion of the needle, the insertion point should be cleared of gel.
Cytologic Preparation and Sample Distribution
The optimum processing and slide preparation for FNA of thyroid is a matter of debate. Although there have been attempts to determine the optimum preparatory procedures, there is actually little evidence to support one method over another, as all such studies suffer from interpretation artifact bias. We learn to interpret the artifacts with which we have had to live and we choose those artifacts as the “optimum” even though they may not be so. Trying to determine which processing and slide preparation protocol is optimum is tantamount to trying to ascertain which language is optimal for expression of complex thoughts. It is evident that each person would choose their native language to communicate, and the same is true for choice of processing of FNA specimens. Combine this with the inherent problems of sample diminution that occur in any attempt to study more than one processing method in parallel, and it becomes apparent that the answer to the dilemma of optimal processing and slide preparation protocol is likely to remain elusive.
A critical aspect of this discussion is that each processing and preparatory protocol induces slightly different artifacts. Therefore, one must be cognizant of the processing artifacts found in the sample under examination and realize that the cytologic features described in a sample processed by an alternative technique may not be evident, or not as clearly evident, as in the sample under consideration. For example, the incidence of nuclear grooving and intranuclear inclusion may vary not only from
lesion to lesion but may be influenced by how the sample has been prepared [18]. The optimum processing and preparatory techniques are those that result in the greatest diagnostic accuracy. As a result, each laboratory should analyze its outcomes and use the technique that yields the highest degree of accuracy that is at least comparable to if not greater than that of their peers and the literature. Furthermore, there is no single processing method that will encompass all variations in samples. As in other aspects of pathology, the pathologist must demonstrate a degree of flexibility and alter the processing technique chosen given the material received to optimize recovery of diagnostic information.
lesion to lesion but may be influenced by how the sample has been prepared [18]. The optimum processing and preparatory techniques are those that result in the greatest diagnostic accuracy. As a result, each laboratory should analyze its outcomes and use the technique that yields the highest degree of accuracy that is at least comparable to if not greater than that of their peers and the literature. Furthermore, there is no single processing method that will encompass all variations in samples. As in other aspects of pathology, the pathologist must demonstrate a degree of flexibility and alter the processing technique chosen given the material received to optimize recovery of diagnostic information.
The preparation of direct smears, both air dried and wet fixed in alcohol, gives the greatest amount of information about background constituents and appearance, stromal fragments and their epithelial relations, and intact epithelial structures with minimization of the trauma induced on these structures. However, direct smears are only interpretable when the smears are properly prepared. Sample maldistribution, excessive obscuring blood, profound hemodilution, overly thick smearing, and improper air-drying or wet-fixation will ruin a sample. If direct smears cannot be properly prepared, then a liquid preparation should be employed.
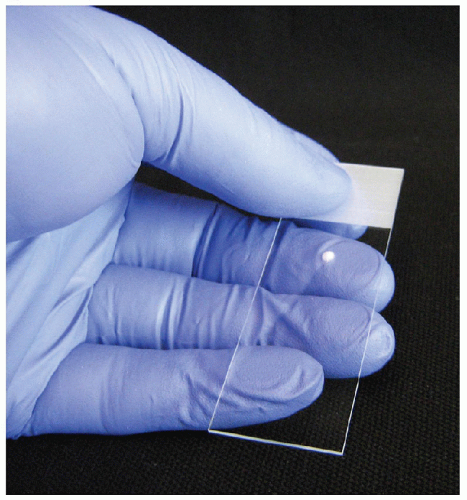
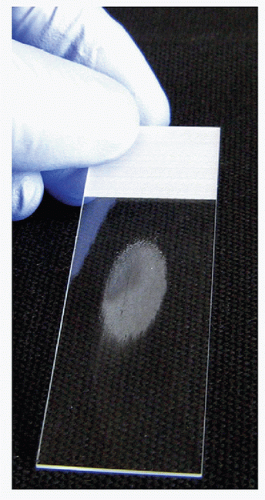
To produce a good-quality direct smear, a single droplet of the FNA sample should be placed on a glass slide approximately 3 cm from the top of the label end and in the center between the two edges. The sample droplet deposited on the glass “sample” slide should have a diameter that does not exceed 2 or 3 mm (Fig. 4.1). With a sample droplet of appropriate size, the sample slide is taken up in one hand and held with the thumb on the surface of the label end with the fingers distributed along the length of the back of the slide to support the entire length (Fig. 4.1). With the second hand, the center of a second “spreading” slide is brought over top of the droplet with the spreading slide perpendicular to the sample slide (Fig. 4.2). The spreading slide is gently lowered toward the sample slide and when the spreading slide contacts the sample droplet, it spreads underneath the spreading slide, mainly by capillary action with very gentle pressure applied. The spreading slide is then drawn down away from the label end along the length of the sample slide, distributing the sample into an oval shape of approximately 3 cm in length without the sample reaching either side of the slide or the end of the sample slide (Fig. 4.3). During the smearing process, very gentle pressure is applied from the spreading slide. The sample is not to be crushed, and the motion of the spreading slide should be a single direct linear stroke. If done correctly, the sample droplet should exhaust before the spreading slide reaches the end of the sample slide and the spreading slide should have virtually no material left on it, with all of the sample deposit onto the sample slide.
How does one generate the sample droplet of 2 to 3 mm on the sample slide from the FNA sample held within the needle bore? Prior to commencing the aspiration procedure, approximately 2 mL of air is drawn into
the syringe. After the FNA strokes are completed, release of the plunger should result in the plunger returning to its starting position with approximately 2 mL of air within the syringe. This amount of air in the syringe allows gentle pressure to be applied to the plunger, generating the force to expel a small drop of sample at the end of the needle. This can then be lightly touched to the sample slide to generate a sample of appropriate size. Since a larger quantity of material will be present in the needle bore, it is best to have multiple sample slides laid out on a tray and simply touch one drop to each slide to produce a number of direct smears. Many descriptions of the aspiration procedure recommend that after completion of the aspiration, that the needle be detached from the syringe and the syringe filled with air to expel the material. This is unnecessary if the syringe is preloaded with 2 mL of air to allow expulsion of the sample. Furthermore, when the syringe is fully filled with air which is pressurized for expulsion,
this generates tremendous force behind the sample which usually results any large quantity of material been squirted onto the slide all at once and exceeding the amount that should be on the slide for direct smears. This situation is worsened if there is any clotting of the sample within the needle prior to expulsion, as greater air pressure will be generated by the aspirator in attempts to get the sample out.
the syringe. After the FNA strokes are completed, release of the plunger should result in the plunger returning to its starting position with approximately 2 mL of air within the syringe. This amount of air in the syringe allows gentle pressure to be applied to the plunger, generating the force to expel a small drop of sample at the end of the needle. This can then be lightly touched to the sample slide to generate a sample of appropriate size. Since a larger quantity of material will be present in the needle bore, it is best to have multiple sample slides laid out on a tray and simply touch one drop to each slide to produce a number of direct smears. Many descriptions of the aspiration procedure recommend that after completion of the aspiration, that the needle be detached from the syringe and the syringe filled with air to expel the material. This is unnecessary if the syringe is preloaded with 2 mL of air to allow expulsion of the sample. Furthermore, when the syringe is fully filled with air which is pressurized for expulsion,
this generates tremendous force behind the sample which usually results any large quantity of material been squirted onto the slide all at once and exceeding the amount that should be on the slide for direct smears. This situation is worsened if there is any clotting of the sample within the needle prior to expulsion, as greater air pressure will be generated by the aspirator in attempts to get the sample out.
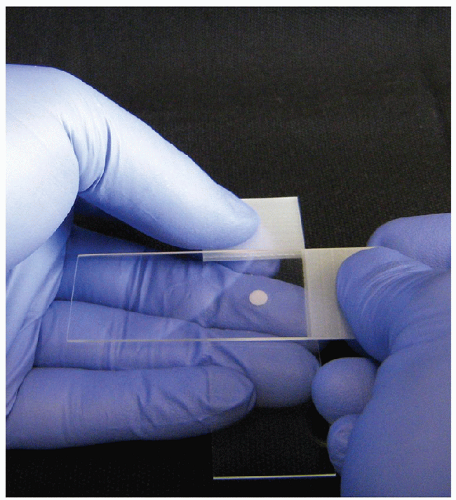 FIGURE 4.2 Smearing technique. With the second hand, the center of a second “spreading” slide is brought over top of the droplet with the spreading slide perpendicular to the sample slide. The spreading slide is gently lowered toward the sample slide, and when the spreading slide contacts the sample droplet, it spreads underneath the spreading slide, mainly by capillary action with very gentle pressure applied. The spreading slide is then drawn down away from the label end along the length of the sample slide, distributing the sample into an oval shape of approximately 3 cm in length (Fig. 4.3). |
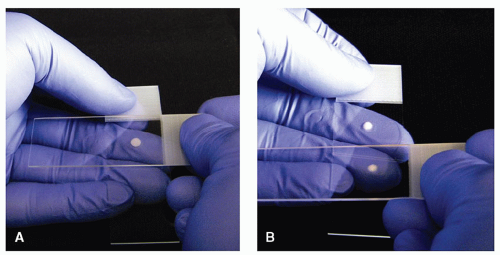
If in exuberance, the aspirator has generated a sample droplet that is too large, the material may be redistributed to multiple slides using a “touch off” procedure. For a “touch off,” the sample slide is held as if to smear the sample. A second sample slide is held as if to smear the sample. Rather than smearing, the second sample slide is brought down to contact the surface of the sample, and by capillary action, a small quantity of sample will be transferred or “touched off” onto the second slide
(Fig. 4.4). This will usually result in a sample droplet that is of appropriate size to allow smearing, and “touch offs” can be performed repetitively to distribute the sample. The “touch off” slides are then smeared as previously described.
(Fig. 4.4). This will usually result in a sample droplet that is of appropriate size to allow smearing, and “touch offs” can be performed repetitively to distribute the sample. The “touch off” slides are then smeared as previously described.
Clearly direct smearing is not applicable to samples in which a large volume of fluid sample has been collected. These samples must be processed as liquid-based preparations or centrifuged with the sediment used for direct smearing once back in the laboratory.
For air-dried smears, a Romanowsky stain is utilized. There are several variants, including Diff Quik, Hemacolor, Field’s stain, and May-Grundwald-Giemsa. All of the variants have slightly different staining characteristics but are similar in many aspects, and the choice of one over another is based on personal preference, rapidity of staining and costs. Wet fixation can be combined with immediate erythrolysis using a modified Carnoy’s solution, a combination of 95% ethanol in varying ratios with a small amount of glacial acetic acid. The acetic acid is an effective erythrolytic agent for unaltered red blood cells introduced into the sample during acquisition but does not destroy old erythrocytes that are indicative of previous hemorrhage. There are variations on wet fixation with some preferring spray fixative and some preferring air drying with rehydration.
Again each approach has its own artifact, and some artifacts are touted to have benefits over others. The wet-fixed direct smears are stained using a modified Papanicolaou stain, which again is quite variable from formulation to formulation and laboratory to laboratory.
Again each approach has its own artifact, and some artifacts are touted to have benefits over others. The wet-fixed direct smears are stained using a modified Papanicolaou stain, which again is quite variable from formulation to formulation and laboratory to laboratory.
Even after making direct smears, there is typically some residual sample in the bore of the needle. This is collected by inserting the needle into a fluid, drawing the fluid into the syringe and expelling the contents into the container. The rinse procedure may be repeated to clear the sample from the needle. The entire FNA sample also may be placed into the needle rinse, particularly when a large volume of fluid is recovered. The fluid used for a needle rinse may be a balanced salt solution, tissue culture media, or fixative. The choice of the rinse solution is dictated by how the needle rinse will be used. It cytomorphologic evaluation is required, then a fixative solution may be chosen; many fixatives have the added advantage of containing an erythrolytic agent to help reduce contaminating blood. A variety of processing techniques are available to allow cytomorphologic examination of the needle rinse including cytocentrifugation, ThinPrep [18, 19, 20, 21, 22, 23, 24, 25], direct smears from the centrifuged sentiment, filtration methods [26], and preparation of a paraffin cell block [27, 28]. The variations and permutations of these techniques are too great to be detailed here, and the reader is directed to the literature listed in the references for more details.
The needle rinse is a highly valuable commodity as it is the substrate upon which ancillary testing can be performed. On a basic level, a formalin-fixed paraffin-embedded cell block produced from the needle
rinse can be employed for immunoperoxidase studies. As another example, the needle rinse from an FNA showing an abnormal lymphoid population may be collected into a sterile salt solution and becomes an ideal cell suspension for immunophenotyping by flow cytometry. Finally, the needle rinsed either in a fresh salt solution or recovered from a fixative may be used for molecular analysis. It is evident, therefore, that the ideal handling of a specimen should include capture of this material.
rinse can be employed for immunoperoxidase studies. As another example, the needle rinse from an FNA showing an abnormal lymphoid population may be collected into a sterile salt solution and becomes an ideal cell suspension for immunophenotyping by flow cytometry. Finally, the needle rinsed either in a fresh salt solution or recovered from a fixative may be used for molecular analysis. It is evident, therefore, that the ideal handling of a specimen should include capture of this material.
Evaluation of a Thyroid FNA
ADEQUACY OF THE SAMPLE. In the preceding sections, we reviewed the process to obtain and process an adequate specimen. The importance of this cannot be overemphasized. No matter how great the interpretive skills of the pathologist, if the sample obtained is inadequate, or has been grossly perturbed, no accurate interpretation will be possible. Thus, no discussion of interpretations can be undertaken without consideration of the determination of sample adequacy.
There are few areas in cytology that engender as much controversy as the assessment of adequacy of a thyroid FNA by pathologic criteria. Here, we emphasize the qualifying statement of pathologic criteria. Unquestionably, the assessment of adequacy goes beyond the simple pathologic features that are evident on the slides. However, many situations arise where the pathologist is not the aspirator or is unable to obtain the clinical or imaging information, and the pathologist is left with no choice but to use pathologic criteria to determine if the sample is adequate. The pathological assessment of adequacy is tremendously flawed. Every assessment of every pathologic sample begins with the determination of whether or not the sample is representative of the lesion in question. Yet, it is impossible to determine if a sample is adequate pathologically unless one can definitively diagnose a pathologic lesion, and this lesion is sufficiently unique in its characteristics, so that reasonable differential diagnoses can be removed from consideration. Thus, when abnormal cells are present in the sample, some degree of adequacy is assured. But when one is faced with relatively bland follicular epithelial cells, the assessment of adequacy becomes problematic. How does one determine if the benign follicular epithelium is representative of the targeted lesion and how much of this epithelium must be assessed to assure a level of clinical significance to the sample?
In general, we acknowledge that if there are no cells present in the sample (acellular), a pathologic interpretation cannot be rendered. Clearly when there is abundant cellular material, most pathologists feel relatively confident that this lesion has likely been sampled, and the interpretation is likely to be representative of the lesion. But somewhere in-between, a sample is probably becoming inadequate when the cellularity is low. This is the premise behind the concept of epithelial quantitation as a means of determining sample adequacy [29]. But where this premise fails is that no one has established nor will anyone ever be able to establish how much
epithelium is really required to assess the nature of the lesion. Furthermore, some would argue that epithelium itself is not always required to assess adequacy. For example, the identification of constituents of a cystic lesion, including proteinaceous material, possible blood, and macrophages, both regular and hemosiderin laden, is indicative of a cystic lesion and therefore provides diagnostic information. Clearly this is true; however, we know cystic lesions of the thyroid may be benign or malignant. The aspiration procedure itself is often indicative that the lesion is cystic by the recovery of some significant volume of fluid, and thus, the sample has been sent for pathologic evaluation not to determine if it is a cystic lesion, but to ascertain whether it is a benign cyst or a malignant cystic tumor. The distinction between these two entities depends on examination of the epithelium that lines the cystic lesion. Therefore, we are back to an evaluation of a number of epithelial cells to determine if the sample is adequate. Several definitions have been employed using different cut-off points for epithelial quantitation [10, 29, 30, 31, 32, 33]. As none of these definitions has adequate scientific investigation or proof behind them, we leave it to the reader to review this material on their own and establish what constitutes an adequate sample. It must be remembered that epithelial quantitation will fail in a number of benign lesions such as a colloid nodule, in which it has been recommended that no minimum number of epithelial cells is required to consider a sample adequate [13].
epithelium is really required to assess the nature of the lesion. Furthermore, some would argue that epithelium itself is not always required to assess adequacy. For example, the identification of constituents of a cystic lesion, including proteinaceous material, possible blood, and macrophages, both regular and hemosiderin laden, is indicative of a cystic lesion and therefore provides diagnostic information. Clearly this is true; however, we know cystic lesions of the thyroid may be benign or malignant. The aspiration procedure itself is often indicative that the lesion is cystic by the recovery of some significant volume of fluid, and thus, the sample has been sent for pathologic evaluation not to determine if it is a cystic lesion, but to ascertain whether it is a benign cyst or a malignant cystic tumor. The distinction between these two entities depends on examination of the epithelium that lines the cystic lesion. Therefore, we are back to an evaluation of a number of epithelial cells to determine if the sample is adequate. Several definitions have been employed using different cut-off points for epithelial quantitation [10, 29, 30, 31, 32, 33]. As none of these definitions has adequate scientific investigation or proof behind them, we leave it to the reader to review this material on their own and establish what constitutes an adequate sample. It must be remembered that epithelial quantitation will fail in a number of benign lesions such as a colloid nodule, in which it has been recommended that no minimum number of epithelial cells is required to consider a sample adequate [13].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree