Follicular Lesions
FOLLICULAR NODULAR DISEASE
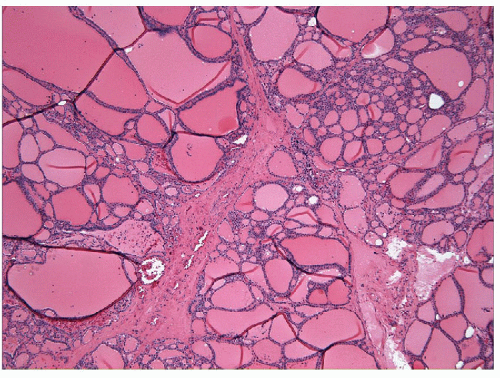
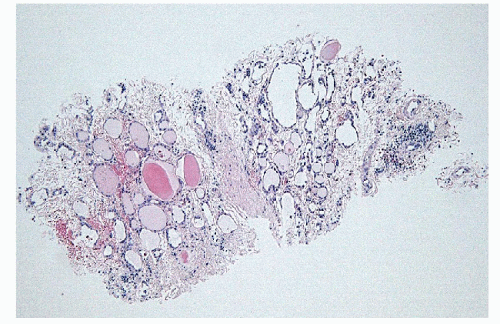
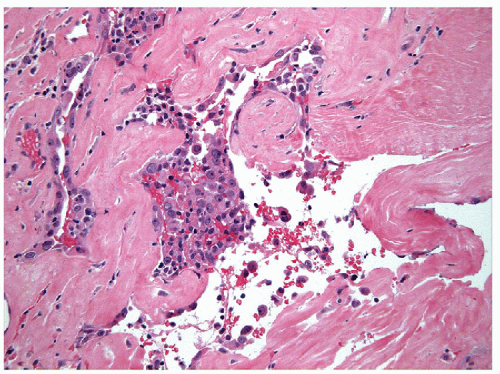
The most common disorder of the thyroid is the presence of numerous follicular nodules with heterogeneous architecture and cytology. These lesions may be small and clinically insignificant, or they may become large and complex, giving rise to the entity known as sporadic nodular goiter (Fig. 10.1, e-Figs. 10.1, 10.2, 10.3, 10.4).
The pathogenesis of this common disorder is unknown. The presence of multifocal, poorly delineated proliferative lesions has been interpreted as indicative of a hyperplastic phenomenon and several pathogenetic mechanisms, including autoimmunity and altered hormonal regulation, have been proposed [1, 2, 3, 4]. The features supporting a hyperplastic etiology include incomplete encapsulation, poor demarcation from internodular tissue, and heterogeneity of follicular architecture. However, the common identification of large encapsulated lesions with relatively monotonous architecture within these nodular glands make distinction of hyperplasia from adenoma difficult. The confusion surrounding this disorder is reflected in the terminology applied to the diagnosis of the lesions. They are called “colloid nodules,” but in many instances, they are not colloid rich. They are called “hyperplastic” nodules, but molecular studies have indicated that they are monoclonal proliferations. Therefore, they represent benign neoplasms and may be more appropriately called adenomas [2, 5], similar to solitary follicular nodules [6, 7]. In fact, the hyperfunctioning nodules that give rise to Plummer’s disease in multinodular thyroids are also now known to be clonal benign neoplasms with activating mutations of the TSH receptor or GNAS [8, 9]. These lesions are discussed in Chapter 9, the section on papillary lesions, because they usually have papillary architecture. The terminology “adenomatous” or “adenomatoid” nodule, although vague and lacking conviction, may be the most appropriate at this point until further progress is made in the understanding of their pathogenesis. However, we have implemented the term “follicular nodular disease” as a descriptive terminology that avoid connotations of hyperplasia or neoplasia which cannot be substantiated.
Pathologists should be aware that the thyroid is a site where progression from hyperplasia to neoplasia occurs and where additional genetic events may give rise to malignant transformation. This is rarely recognized
clinically, since the vast majority of nodular goiters remain entirely clinically benign, but the occasional emergence of malignancy in multinodular goiter supports this concept.
clinically, since the vast majority of nodular goiters remain entirely clinically benign, but the occasional emergence of malignancy in multinodular goiter supports this concept.
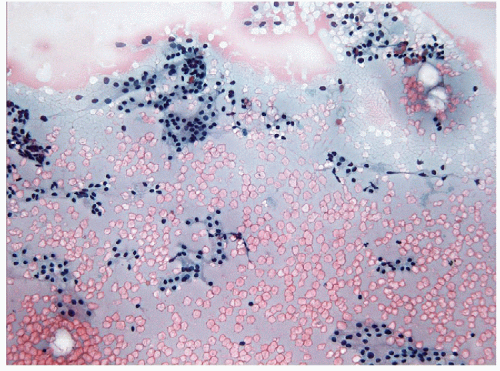
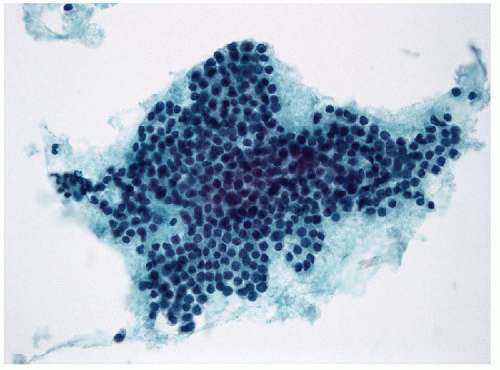
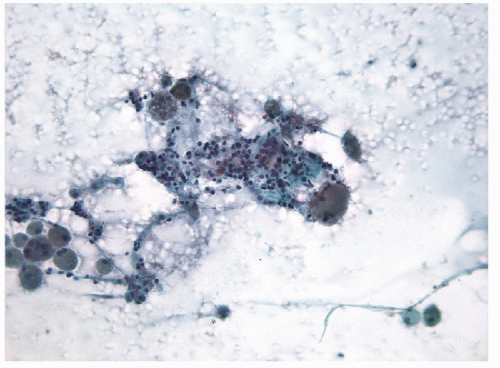
Fine needle aspiration (FNA) and biopsy of these lesions identifies follicular epithelium in a combination of macrofollicular and microfollicular architectures [10]. The macrofollicular architecture in FNA is recognized by monolayered sheets and less commonly, as intact macrofollicles. The amount of colloid is variable, but often abundant, and the colloid itself is often watery in consistency (Fig. 10.2, e-Figs. 10.5 and 10.7). Because of this, FNAs processed by ThinPrep usually show little colloid as it is sufficiently watery to be lost through the filter. When identified, the abundance of colloid suggests a benign process, but this can be misleading, since follicular variant papillary carcinomas can also have abundant colloid and the absence of colloid may be the result of processing techniques such as monolayer preparations. The critical feature is the presence of simple monolayered sheets of epithelium composed of evenly distributed and well-structured follicular epithelial cells with small round nuclei, a fine chromatin pattern and relatively inconspicuous nucleoli [11, 12] (Figs. 10.3 and 10.4, e-Figs. 10.6 and 10.7). In direct smears, the cytoplasm appears to be moderately abundant, but seems less voluminous in ThinPrep preparations as a result of rounding-up of the cells during liquid fixation. Oncocytic change is relatively common and the cytoplasm may show numerous, small blue-black granules known as
“paravacuolar granules” [12] (e-Fig. 10.8) that represent lysosomes containing hemosiderin and lipofuscin pigment.
“paravacuolar granules” [12] (e-Fig. 10.8) that represent lysosomes containing hemosiderin and lipofuscin pigment.
There are no cytologic features of the epithelial cells that allow distinction of “hyperplastic” follicular epithelium from normal “non-hyperplastic”
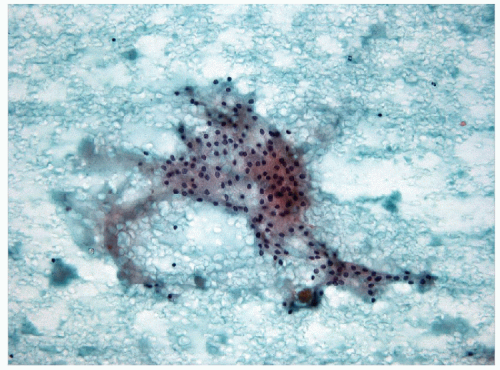
epithelium. Thus, cytology has utilized two secondary findings that may occur in follicular nodular disease. The first of these is a relative abundance of colloid as discussed. Again it must be remembered that the colloid seen on the slides may under-represent the colloid content of the lesion. The second is the finding of remote hemorrhage or “degenerative changes” as indicated by the presence of “old” red blood cells, resistant to erythrolytic agents and the presence of hemosiderin-laden macrophages. Nodular lesions may undergo hemorrhagic cystic change, therefore the finding of remote hemorrhage may suggest the presence of a lesion rather than normal tissue, with the very significant caveat that the epithelium present must not be malignant (Fig. 10.5). Sample cellularity has occasionally been suggested as a means to ascertain whether a lesion is hyperplastic. However, the cellularity of a FNA sample is far more dependent on the skill of the aspirator and the quality of the aspiration than on the nature of the lesion, so that sample cellularity cannot be used to reliably establish the pathological nature of the lesion. In fact, in many biopsies of thyroid follicular nodular disease, the FNA is simply labeled as “benign thyroid tissue.”
epithelium. Thus, cytology has utilized two secondary findings that may occur in follicular nodular disease. The first of these is a relative abundance of colloid as discussed. Again it must be remembered that the colloid seen on the slides may under-represent the colloid content of the lesion. The second is the finding of remote hemorrhage or “degenerative changes” as indicated by the presence of “old” red blood cells, resistant to erythrolytic agents and the presence of hemosiderin-laden macrophages. Nodular lesions may undergo hemorrhagic cystic change, therefore the finding of remote hemorrhage may suggest the presence of a lesion rather than normal tissue, with the very significant caveat that the epithelium present must not be malignant (Fig. 10.5). Sample cellularity has occasionally been suggested as a means to ascertain whether a lesion is hyperplastic. However, the cellularity of a FNA sample is far more dependent on the skill of the aspirator and the quality of the aspiration than on the nature of the lesion, so that sample cellularity cannot be used to reliably establish the pathological nature of the lesion. In fact, in many biopsies of thyroid follicular nodular disease, the FNA is simply labeled as “benign thyroid tissue.”
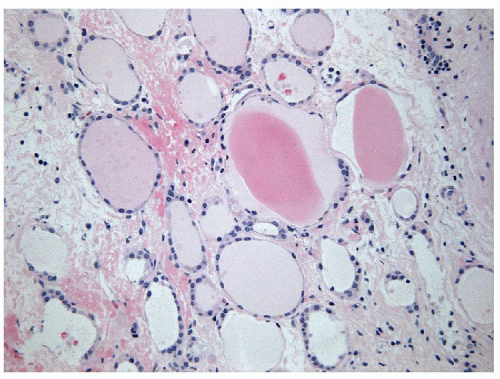
Core biopsies also reveal variably-sized follicles lined by a single layer of simple follicular epithelial cells with rounded nuclei that have vesicular chromatin and inconspicuous nucleoli (Figs. 10.6 and 10.7). Macrophages, usually filled with hemosiderin are also noted; however, their number depends upon the presence or absence of secondary degenerative changes or a cystic component [12, 13, 14]. Fibrosis may
be seen but is usually not abundant and tends to be irregular, due to degeneration.
be seen but is usually not abundant and tends to be irregular, due to degeneration.
The distinction of multinodular disease from follicular neoplasia is an area of controversy. The size of follicles has been thought to be significant
in the assessment of these nodules. Hyperplasia was thought to be characterized by macrofollicular lesions with abundant colloid whereas neoplasia was thought to be a more cellular process with microfollicular architecture. Clonality studies have disproved this hypothesis and there is no correlation between clonality as a reflection of neoplasia and structural morphology of these follicular cell proliferations [5]. Indeed, based on the clonality data, the distinction between follicular nodular disease and follicular adenoma is likely to be entirely academic unless there is a possibility of malignancy. However, as discussed in the next section, the distinction between adenoma and carcinoma is not possible based on cytologic features alone, resulting in a clinical dilemma about the correct approach to the diagnosis of what is clearly a proliferative lesion of thyroid follicular epithelium that lacks clear and unequivocal features of malignancy. The various approaches are reviewed in Chapter 4.
in the assessment of these nodules. Hyperplasia was thought to be characterized by macrofollicular lesions with abundant colloid whereas neoplasia was thought to be a more cellular process with microfollicular architecture. Clonality studies have disproved this hypothesis and there is no correlation between clonality as a reflection of neoplasia and structural morphology of these follicular cell proliferations [5]. Indeed, based on the clonality data, the distinction between follicular nodular disease and follicular adenoma is likely to be entirely academic unless there is a possibility of malignancy. However, as discussed in the next section, the distinction between adenoma and carcinoma is not possible based on cytologic features alone, resulting in a clinical dilemma about the correct approach to the diagnosis of what is clearly a proliferative lesion of thyroid follicular epithelium that lacks clear and unequivocal features of malignancy. The various approaches are reviewed in Chapter 4.
FOLLICULAR NEOPLASIA
Follicular adenoma, follicular variant papillary carcinoma, and follicular carcinoma are all well-differentiated neoplasms of thyroid follicular epithelium. These diagnoses represent the most difficult and controversial thyroid pathology because of their extensive overlap. The distinctions are based on evaluation of nuclear alterations and identification of capsular and vascular invasion. The inter- and intraobserver variability in the interpretation of these features is the most significant in the field of histopathology [15, 16, 17, 18, 19].
These tumors are virtually indistinguishable with respect to their clinical presentation and radiographic appearance. In most cases, the architectural morphology is essentially the same on histology. The distinction between follicular adenoma and follicular carcinoma has been considered possible only by recognition of capsular and/or vascular invasion or metastasis [20, 21]. The follicular variant of papillary carcinoma was defined as a lesion with follicular architecture but with the nuclear features of papillary carcinoma [22] (see detailed description in Chapter 9). This entity has been recognized with increasing frequency [23, 24, 25] and at the expense of lesions classified as follicular carcinoma [26] likely because of increasing recognition of nuclear atypia. In areas of endemic goiter, the decrease in follicular carcinoma may be real due to dietary iodine supplementation [26], however, the more widespread change in incidence is attributable to recognition of the nuclear features that change the criteria for diagnosis [15, 27]. Indeed, the concept of nuclear atypia as a marker of follicular epithelial malignancy should be reconsidered; rather than signifying papillary carcinoma, it may also be reflective of follicular carcinoma [28, 29].
The similarities between these lesions have been emphasized by the results of molecular analyses of thyroid tumors. All three of these types of follicular neoplasia harbor RAS mutations, mainly NRAS but also HRAS and KRAS, as their primary genetic alteration [30, 31, 32, 33]. Mutations in EIF1AX are also characteristic of follicular-patterned neoplasms [34]. The BRAFV600E mutation which is characteristic of classical papillary thyroid carcinoma (PTC) is not identified in these tumors; instead, tumors with follicular architecture have been reported to display BRAFK601E mutations [35, 36, 37, 38]. Rare follicular variant papillary carcinomas harbor ret/PTC rearrangements [33]. Some harbor a unique rearrangement involving the peroxisome proliferator activated receptor gamma (PPARγ). PPARγ, encoded by PPARG (3p25), is a member of the steroid nuclear hormone receptor superfamily that forms heterodimers with retinoid X receptor; it is best known for its differentiating effects on adipocytes and insulin-mediated metabolic functions. PAX8-PPARG rearrangements were first identified in thyroid neoplasms with a cytogenetically detectable translocation t(2;3)(q13;p25) that generates a chimeric gene encoding the DNA binding domain of the thyroid-specific transcription factor paired-box gene (PAX8) and domains A-F of PPARG [39]. The PPARG rearrangement acts through a dominant-negative effect on the transcriptional activity of wild-type PPARγ [39]. The fusion oncoprotein is thought to contribute to malignant transformation by targeting several cellular pathways, at least some of which are normally engaged by PPARγ. The rearrangement was initially reported to be restricted to follicular thyroid carcinoma with the initial suggestion that it correlates with an angioinvasive phenotype [40, 41]. Subsequently the same rearrangement was found in follicular adenomas and in follicular variant PTC [33, 42, 43, 44], again reflective of the overlap between these various entities. Other molecular causes of follicular thyroid
neoplasia include PTEN mutations in patients with the PTEN-hamartoma tumor syndrome where multifocal lesions are characteristic [45] and rare mutations reported in STK11 [46].
neoplasia include PTEN mutations in patients with the PTEN-hamartoma tumor syndrome where multifocal lesions are characteristic [45] and rare mutations reported in STK11 [46].
The diagnosis of follicular adenoma applies to a neoplasm that has mature, well-formed follicular architecture exhibits no evidence of nuclear enlargement, irregularity or other features of papillary carcinoma, and has no invasive behavior. Follicular adenomas have been classified according to the size of follicles, abundance of colloid, and degree of cellularity [20, 47], but there is no clinical significance to the distinction of macrofollicular, microfollicular, fetal, or embryonal lesions as described in the older literature. Cytologic atypia in follicular neoplasms is always worrisome, but in this situation the major focus should be on the identification of follicular variant papillary carcinoma through the recognition of the nuclear features of papillary carcinoma. The presence of the cytologic features of papillary carcinoma should indicate that diagnosis, however, the identification of these nuclear features is inconsistently achieved and there has not been consensus on the minimum set of features that constitutes a definitive diagnosis, thus leading to a highly subjective area that exhibits the most inconsistency in diagnostic pathology [15]. The identification of mitotic figures, nuclear hyperchromasia or other cellular atypia that does not fulfil the criteria for papillary carcinoma is considered to be inconsequential. This so-called “endocrine atypia” [20, 21, 48, 49, 50] has no implication for malignancy in well-differentiated thyroid follicular epithelium.
The diagnosis of follicular carcinoma is applied to a lesion of the same architecture and cytology but that has unequivocal evidence of invasion. It is well recognized that invasion cannot be reliably identified in FNA or core biopsy material. Therefore, these lesions are diagnosed as follicular neoplasia and the diagnosis of follicular adenoma or carcinoma can only be rendered for definitive diagnosis on thorough examination of a resection specimen.
Follicular carcinomas are divided into groups that reflect the biology of tumor growth and metastasis. These include widely invasive, minimally invasive, and angioinvasive lesions. Widely invasive follicular carcinomas are relatively easy to recognize, but the other two variants require careful microscopic examination. Some authors consider both categories to be “minimally” invasive because of the microscopic nature of the invasion [51, 52], but the biological differences, discussed later, make it evident that these represent two very different risk and prognostic categories.
Widely invasive follicular carcinomas may be recognized radiologically or on ultrasound and are usually identifiable as invasive grossly. They are certainly not difficult to recognize as invasive microscopically; there is usually widespread invasion of surrounding parenchyma that may extend to become extrathyroidal extension [53] and resection margin involvement. They carry a poor prognosis with 25% to 45% 10-year survival [54, 55, 56]. In our experience, these are rare and tumors of follicular cell derivation that are widely invasive tend to be poorly differentiated carcinomas (see Chapter 11).
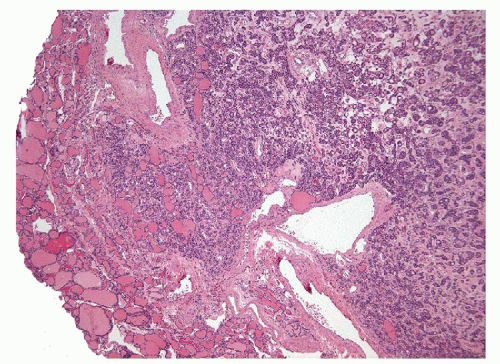
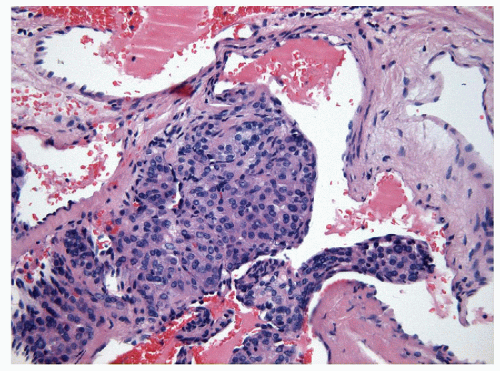
Minimally invasive follicular carcinoma with invasion into and superficially through the capsule (Figs. 10.8, 10.9, 10.10, e-Fig. 10.9) occurs in patients who are about 10 years younger than those with widely infiltrative carcinomas and it has been suggested that encapsulated follicular carcinoma is a
precursor of the widely invasive lesion [57]. Minimally invasive carcinomas have 10-year survival rates of 70% to 100% [58, 59, 60]. Clinically and radiologically they mimic follicular adenomas. The distinction between this type of follicular carcinoma and follicular adenoma requires histologic evaluation with careful evaluation of the capsule [61]. It is important to note that the criteria for invasion are not always applied in the same way [17, 19] resulting in discrepant diagnoses; invasion into the capsule is considered sufficient by some pathologists while others require full thickness invasion into the surrounding parenchyma. The pattern of invasion, whether parallel or perpendicular to the capsule, is important when distinguishing true invasion from artifact caused by previous biopsy [19]. Importantly, the determination of capsular invasion is not possible on FNA or core biopsy.
precursor of the widely invasive lesion [57]. Minimally invasive carcinomas have 10-year survival rates of 70% to 100% [58, 59, 60]. Clinically and radiologically they mimic follicular adenomas. The distinction between this type of follicular carcinoma and follicular adenoma requires histologic evaluation with careful evaluation of the capsule [61]. It is important to note that the criteria for invasion are not always applied in the same way [17, 19] resulting in discrepant diagnoses; invasion into the capsule is considered sufficient by some pathologists while others require full thickness invasion into the surrounding parenchyma. The pattern of invasion, whether parallel or perpendicular to the capsule, is important when distinguishing true invasion from artifact caused by previous biopsy [19]. Importantly, the determination of capsular invasion is not possible on FNA or core biopsy.
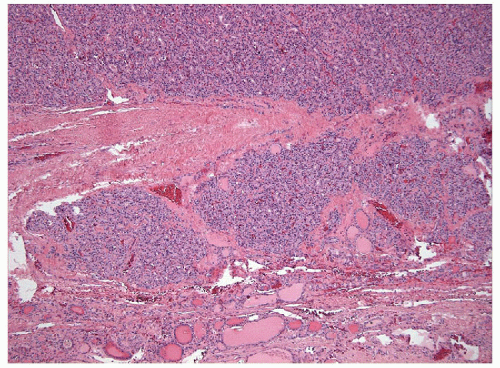 FIGURE 10.9 Minimally invasive follicular carcinoma. The tumor invades the thyroid along the outer edge of the tumor capsule (hematoxylin & eosin stain). |
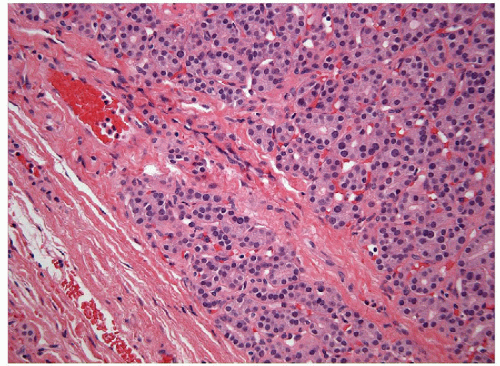 FIGURE 10.10 Capsular Invasion. A follicular neoplasm may show an island of neoplastic cells surrounded by fibrous bands of the capsule that may constitute invasion into the tumor capsule. However, the irregular nature of the tumor capsule makes this assessment difficult and capsular invasion is often not accepted unless full thickness penetration is evident as in Figures 10.8 and 10.9 (hematoxylin & eosin stain). |
The concept of unencapsulated follicular carcinoma was raised by the identification of tumors that lack a capsule. In one report of four such cases, one patient developed metastases, and this gave rise to citations of a 25% metastatic rate by such lesions [62]. However, this has not been substantiated in larger series and this concept has largely been abandoned. Invasion in such lesions should be established by infiltration of the surrounding parenchyma.
Angioinvasive follicular carcinomas are aggressive and require management accordingly. While vascular invasion is more reliable for the diagnosis of malignancy, again the criteria are controversial [18, 19]. This controversial area also requires complete histological evaluation and
cannot be reliably determined on biopsy. Vascular invasion cannot be evaluated within the tumor and therefore the circumference of the lesion is the site that warrants careful examination. Bulging of tumor under endothelium does not qualify as vascular invasion if the endothelium is intact, however, this is often misinterpreted as invasion, giving rise to reports suggesting that angioinvasion does not alter prognosis [51, 63]. Nests of tumor cells within an endothelial lumen generally are accepted as representing invasion. However, artifactual implantation of tumor cells into blood vessels can occur during biopsy, surgery, or sectioning, particularly in endocrine tissues that have thin fenestrated endothelium [18]. Therefore, the identification of tumor cells invading through a vessel wall and thrombus adherent to intravascular tumor is required to distinguish true invasion from artifact (Figs. 10.11 and 10.12). The dominant determinant of cause-specific mortality in patients with follicular carcinoma is the presence of distant metastases [64, 65, 66] which is a reflection of angioinvasion when defined appropriately [18, 52, 67].
cannot be reliably determined on biopsy. Vascular invasion cannot be evaluated within the tumor and therefore the circumference of the lesion is the site that warrants careful examination. Bulging of tumor under endothelium does not qualify as vascular invasion if the endothelium is intact, however, this is often misinterpreted as invasion, giving rise to reports suggesting that angioinvasion does not alter prognosis [51, 63]. Nests of tumor cells within an endothelial lumen generally are accepted as representing invasion. However, artifactual implantation of tumor cells into blood vessels can occur during biopsy, surgery, or sectioning, particularly in endocrine tissues that have thin fenestrated endothelium [18]. Therefore, the identification of tumor cells invading through a vessel wall and thrombus adherent to intravascular tumor is required to distinguish true invasion from artifact (Figs. 10.11 and 10.12). The dominant determinant of cause-specific mortality in patients with follicular carcinoma is the presence of distant metastases [64, 65, 66] which is a reflection of angioinvasion when defined appropriately [18, 52, 67].
The follicular variant of papillary carcinoma encompasses a spectrum of lesions with follicular architecture and nuclear atypia. These include well delineated, non-invasive, and sometimes completely encapsulated neoplasms, delineated but locally invasive malignancies, as well as infiltrative tumors that have an aggressive growth pattern and rare angioinvasive cancers. The biological and clinical behaviors of these various lesions are different. Initial reports of this entity included aggressive lesions that developed metastases, leading to the impression that this variant is more aggressive than the classical form [23, 68]. However, prior to the 1980s, most of these
lesions were diagnosed as follicular adenomas and atypical adenomas, and most patients did well with no long-term sequelae following limited surgery. Recent data including a large meta-analysis of published literature [69] indicate that this variant is more indolent than classical PTC. However, the various growth patterns are also important. It must be emphasized that the identification of even a single papilla, even small abortive papillae, as well as the presence of true psammoma bodies should result in the diagnosis of classical variant papillary carcinoma, since these lesions have a more “BRAF-like” molecular signature [33], behavior and outcome. Most infiltrative follicular variant papillary carcinomas fall into this category [70].
lesions were diagnosed as follicular adenomas and atypical adenomas, and most patients did well with no long-term sequelae following limited surgery. Recent data including a large meta-analysis of published literature [69] indicate that this variant is more indolent than classical PTC. However, the various growth patterns are also important. It must be emphasized that the identification of even a single papilla, even small abortive papillae, as well as the presence of true psammoma bodies should result in the diagnosis of classical variant papillary carcinoma, since these lesions have a more “BRAF-like” molecular signature [33], behavior and outcome. Most infiltrative follicular variant papillary carcinomas fall into this category [70].
Well delineated and encapsulated neoplasms with nuclear features of papillary carcinoma have been the subject of extensive investigation. Prior to 1977, these lesions were classified as “follicular adenoma.” The recognition of metastatic potential, usually to local lymph nodes, resulted in reclassification of any lesion with nuclear features of papillary carcinoma as “follicular variant papillary carcinoma” [23]. However, in well delineated or encapsulated tumors that do not show invasive behavior, the incidence of metastasis is small, and the risk incurred is not of major clinical significance, since loco-regional nodal spread does not alter long-term outcome. Therefore, these are considered to be low-risk lesions [71, 72, 73]. In an effort to modulate clinical management by altering terminology, there has been a proposal formulated to rename these lesions “non-invasive follicular thyroid neoplasm with papillary-like nuclear features” (NIFTP) [74].
The identification of any capsular invasion or infiltration into surrounding parenchyma in a well delineated follicular variant papillary carcinoma should indicate the potential for metastatic spread. Usually this involves loco-regional lymph nodes. The behavior of tumors with lymphatic invasion is also relatively indolent with similar local spread to lymph nodes. In contrast, the identification of angioinvasion, if applied using proper criteria [18], portends higher risk of distant metastases.
On FNA, many follicular variant papillary carcinomas may be recognized as papillary carcinoma, but not necessarily of the follicular subtype [75, 76, 77, 78]. Although it has been suggested that this variant might be distinguishable on FNA [75, 79, 80], no features that can specifically identify the follicular variant have received acceptance [78, 81, 82, 83, 84]. In fact, in the follicular variant of papillary carcinoma, the nuclear features of papillary carcinoma are often more subtle [85] and, in particular, intranuclear inclusions may be less common [86, 87]. This may account for a high frequency of diagnoses of “suspicious for papillary carcinoma” [85] or “follicular neoplasm” [76, 84, 85, 87, 88] and a propensity for misdiagnosis as a benign lesion [89, 90, 91]. The impact of the change in terminology of the non-invasive lesions to a non-cancer diagnosis will be significant, reducing the rate of malignancy of nearly 50% in the category of suspicious lesions [92].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree