Insulinomas are the most common type of functional pancreatic neuroendocrine neoplasm. They tend to be small (< 2 cm), solitary, benign lesions that can arise anywhere within the pancreas and can be associated with MEN1 (although less commonly than gastrinomas). The diagnosis of insulinoma can be made by the classic Whipple triad of clinical findings: (1) symptoms of hypoglycemia during monitored fasting or exercise; (2) blood glucose levels of less than 50 mg/dL during these symptomatic episodes; and (3) relief of these symptoms following administration of glucose. To detect the Whipple triad, patients with suspected insulinoma are closely monitored during a 72-hour inpatient fast. Following measurement of baseline serum glucose and insulin levels, blood glucose levels are measured every 2 hours, and insulin levels are measured at the onset of hypoglycemic symptoms or when blood glucose levels become lower than 50 mg/dL. Nearly all patients with insulinoma will have inappropriately elevated plasma insulin levels (> 5 μU/mL) in the setting of hypoglycemia. Elevated levels of C peptide and proinsulin are also typically present and help to differentiate insulinoma from factitious hypoglycemia; the onset of symptoms during fasting helps to differentiate insulinoma from postprandial reactive hypoglycemia.
Gastrinomas are the second most common type of functional pancreatic neuroendocrine neoplasm. Unlike insulinomas, 90% of gastrinomas are found within the gastrinoma triangle: an anatomical region defined by the cystic and common bile ducts superiorly, the second and third portions of the duodenum inferiorly, and the pancreatic neck and body medially (Fig. 6.1). Two-thirds of gastrinomas are located within the pancreas, and a third may be found within the duodenum. In sporadic, noninherited forms of gastrinoma, tumors tend to be solitary. However, gastrinomas arising in the context of MEN1 are multifocal and highly prone to recurrence. Because of the ability of gastrin to induce gastric acid secretion, gastrinomas typically present with the classic and often refractory signs and symptoms of Zollinger-Ellison syndrome, consisting of gastroduodenal ulcerations (90%), epigastric pain (75%), diarrhea associated with the large-volume gastric acid secretion (75%), and malabsorption leading to weight loss. The incidence of gastrinoma among patients presenting with peptic ulcer disease has been estimated to be approximately 1%, and this diagnosis should be entertained in patients presenting with advanced or refractory peptic ulcer disease. Unlike insulinomas, gastrinomas are often malignant, with about 50% of patients presenting with evidence of hepatic metastases.
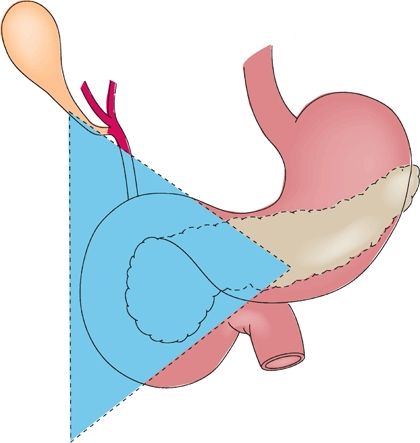
FIGURE 6.1 Most gastrinomas are found within the gastrinoma triangle. (From: Mulholland MW, Lillemoe KD, Doherty GM, et al. Greenfield’s surgery: scientific principles & practice, 5th ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2011.)
Patients with suspected gastrinoma should undergo serum gastrin measurement. The upper limit of normal gastrin level is approximately 100 pg/mL; serum gastrin levels ten times greater than the upper limit of normal in the presence of gastric pH less than 5.0 are diagnostic of gastrinoma. However, patients with gastrinoma often present with more subtle degrees of hypergastrinemia. The differential diagnosis for moderate hypergastrinemia includes achlorhydria (as can occur with proton pump inhibitor therapy), retained antrum following partial gastrectomy, gastric outlet obstruction, and renal insufficiency. The secretin stimulation test is a means of increasing the sensitivity of detecting gastrinoma. Following intravenous administration of 2 μg/kg secretin, a paradoxical rise in serum gastrin by greater than 200 pg/mL is considered positive for gastrinoma. Similarly, a paradoxical rise in serum gastrin of greater than 50% following infusion of calcium gluconate is also indicative of gastrinoma.
Unlike insulinomas and gastrinomas, glucagonomas are characteristically larger lesions that tend to be localized along the body and tail of the pancreas. Like gastrinomas, they exhibit a proclivity for aggressive behavior, with approximately 50% of cases harboring evidence of distant metastatic disease. The ability of glucagon to promote gluconeogenesis and amino acid oxidation explains the characteristic clinical findings of diabetes (resulting from hyperglycemia) and dermatitis (resulting from amino acid deficiency). Skin manifestations of dermatitis include necrolytic migratory erythema, characterized by painful blistering plaques along the face, abdomen, lower extremities, and mucous membranes. Interestingly, up to a third of patients with glucagonoma develop deep venous thrombosis, warranting consideration of anticoagulation therapy.
Serum glucagon levels greater than 1,000 pg/mL can be diagnostic for glucagonoma. However, as with gastrinoma, more subtle elevations can be seen in patients with glucagonoma. Unlike gastrinoma, provocative testing is not available to help distinguish glucagonoma from potentially confounding conditions that can induce hyperglucagonemia such as pancreatitis, sepsis, Cushing syndrome, fasting, renal insufficiency, and hepatic failure.
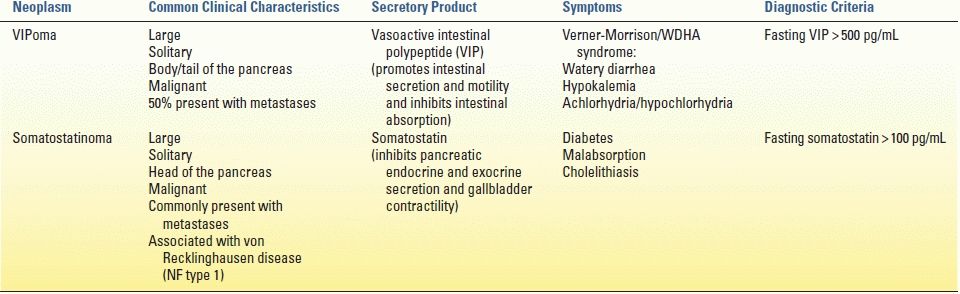
Like glucagonomas, VIPomas often present as large, solitary lesions localized to the pancreatic body and tail and often present with evidence of metastatic disease. Rare extrapancreatic VIPomas have been observed in the colon, bronchus, liver, adrenal glands, and para-aortic ganglia. Vasoactive intestinal polypeptide (VIP) promotes intestinal secretion and motility while inhibiting intestinal absorption of water and electrolytes. As a result, the classic Verner-Morrison syndrome of VIPomas is also referred to by the acronymic WDHA syndrome (consisting of massive watery diarrhea, hypokalemia, and achlorhydria). In patients presenting with profuse secretory diarrhea, the diagnosis of VIPoma can be made by measuring elevated fasting serum VIP levels (typically > 500 pg/mL).
In contrast to glucagonomas and VIPomas, the majority of somatostatinomas are found within the pancreatic head. Like gastrinomas and VIPomas, somatostatinomas can present outside of the pancreas (most commonly in the duodenum and ampulla). Duodenal somatostatinomas are a common manifestation of von Recklinghausen disease (neurofibromatosis type 1). Pancreatic somatostatinomas tend to present as larger tumors with metastatic disease. Somatostatin inhibits secretion of pancreatic insulin and exocrine enzymes and inhibits gallbladder contractility; as a result, somatostatinomas often present with symptoms of diabetes, malabsorption, and cholelithiasis. The diagnosis of somatostatinoma among patients presenting with these symptoms is supported by elevated serum levels of somatostatin greater than 100 pg/mL.
Nonfunctioning pancreatic neuroendocrine neoplasms tend to present evenly throughout all regions of the pancreas. Taken as a whole, they were previously characterized as generally rarer, larger, and more aggressive than functioning pancreatic neuroendocrine neoplasms. However, these characterizations may have been historical artifacts of diagnosis. As more and more pancreatic neuroendocrine neoplasms are being diagnosed incidentally with imaging evaluations rather than by symptoms referable to hormonal production or tumor mass, the prevalence of small, asymptomatic, nonfunctioning pancreatic neuroendocrine neoplasms appears to be increasing. Thus, the extent to which functioning and nonfunctioning pancreatic neuroendocrine neoplasms differ in terms of inherent tumor biology and behavior is unclear. Nonfunctioning lesions can occasionally secrete measurable levels of proteins that do not induce significant symptomatology, but often do not express any known hormones. As a result, elevated serum levels of proteins like pancreatic polypeptide, neuron-specific enolase, and neurotensin can have high specificity but very poor sensitivity in the diagnosis of nonfunctioning pancreatic neuroendocrine neoplasms.
Imaging and Localizing Studies
Diagnostic imaging for pancreatic neuroendocrine neoplasms takes advantage of some of their unique biologic characteristics. The majority of pancreatic neuroendocrine neoplasms are markedly well vascularized. As a result, contrast-enhanced imaging by computed tomography (CT) or magnetic resonance imaging (MRI) should employ both early arterial and delayed portal-phase imaging sequences, as they tend to appear very bright during early arterial-phase administration of contrast (Fig. 6.2) or on portal-phase imaging (Fig. 6.3). Radiographic diagnosis can be further enhanced with MRI, as pancreatic neuroendocrine neoplasms tend to exhibit low signal intensity on T1-weighted imaging and high signal intensity on T2-weighted imaging (Fig. 6.4). These imaging characteristics can also be used in the detection of extrapancreatic metastases.
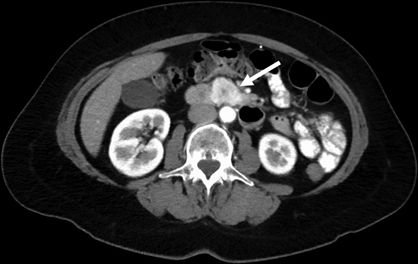
FIGURE 6.2 CT imaging of neuroendocrine neoplasm arising from the uncinate process demonstrates brisk uptake of contrast on early arterial-phase imaging (white arrow).
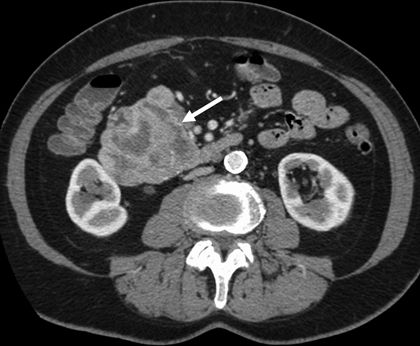
FIGURE 6.3 CT imaging of neuroendocrine neoplasm arising from the pancreatic head demonstrates contrast enhancement on portal-phase imaging with areas of central necrosis (white arrow).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree