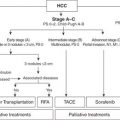
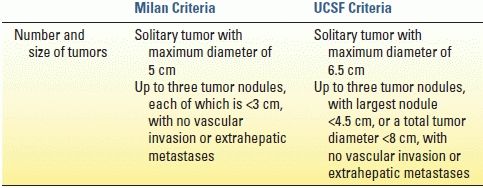
Most contraindications to LT are relative but may include active substance abuse, systemic infection, cancer, HIV infection, poorly managed psychiatric disorders, lack of social support, and significant disease of the cardiopulmonary or neurologic systems. Surveillance for hepatocellular carcinoma (HCC) is mandatory for all patients with cirrhosis; 70% to 90% of all HCC cases arise in the cirrhotic liver. Scoring systems have been developed to determine acceptable HCC tumor volume that still permits LT, the two most common of which are the Milan and the University of California, San Francisco (UCSF) criteria (Table 30.2). Liver-directed therapies for HCC such as transarterial embolization and radiofrequency ablation are useful and serve to control disease burden while awaiting LT; these therapies may also be used to downstage HCC volume to meet transplant criteria.
End-stage liver disease is frequently accompanied by a host of comorbidities that may impact the outcome of the transplant. Hepatorenal syndrome (HRS) is a condition in which chronic liver disease is associated with renal insufficiency. Though some of the effects of severe HRS may be reversed with LT, patients do not generally return to completely normal renal function. Hepatopulmonary syndrome (HPS) results from differences in vascular pressures related to the primary liver disease. The vasodilation resulting from liver failure leads to a direct vasodilatory effect in the lungs, which results in increased blood flow in relation to ventilation and a ventilation–perfusion mismatch. This is seen clinically as a right-to-left shunt, and the patient experiences dyspnea. As with HRS, HPS is an important marker of disease severity, and prognosis is poor without a timely LT. Symptoms of HPS improve markedly with LT.
TABLE 30.2 Criteria for Liver Transplantation in Patients with Hepatocellular Carcinoma
PROCEDURE/TECHNIQUE
Organ Procurement from Deceased Donors
The organ procurement operation from a deceased donor is standardized with little variation among surgeons. Safe preservation of the organs is dependent upon two primary principles: (1) rapid organ exsanguination and vascular flushing with an appropriate preservation solution and (2) rapid organ cooling. After the organs have been prepared in the donor, an aortic cannula is placed that is used to flush the arterial system. Some surgeons choose to place a second cannula in the portal vein (through the inferior mesenteric vein) to flush the portomesenteric system. The abdominal aorta is clamped superior to the celiac trunk and also near the iliac bifurcation to isolate the abdominal organs. The outflow for the blood and preservation solution is generally through the suprahepatic vena cava at its junction with the right atrium. As the clamps are placed and the preservation solution is infused, iced normal saline is placed throughout the abdominal cavity to topically cool the organs. The heart arrests during this process, and the organs are then removed. For living donor partial liver procurement, the liver resection is performed in the donor with dissection completed while vascular inflow and outflow remains intact. Clamps are applied simultaneously, and the donor graft is removed, followed by rapid flushing and cooling (Fig. 30.1A and B). Donation after cardiac death (DCD) is currently used in 4% of deceased donors and requires complete cessation of cardiopulmonary function prior to organ procurement. This necessarily results in several minutes of graft warm ischemia time, which results in inferior outcomes. In all cases, once procured, the liver allograft must be transplanted and reperfused within 12 hours, after which there is an increased risk of complications.
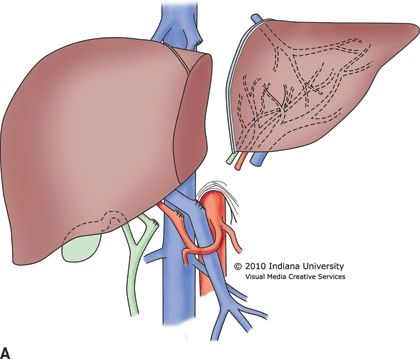
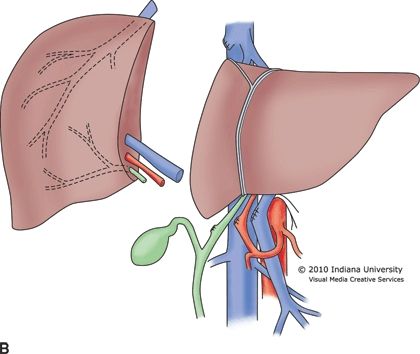
FIGURE 30.1 A. Living donor left lateral segment graft for a pediatric recipient. B. Living donor right lobe graft for an adult recipient.
Liver Transplant Operation
There are three components to the liver transplant operation: (1) preparation of the transplant organ, (2) recipient hepatectomy, and (3) implantation of the graft. Preparation of the graft involves removal of residual tissue from the donor operation including residual diaphragm and pericardium around the vena cava and hepatic veins. The gallbladder is always removed from the donor liver, and the cystic duct is ligated. Accessory hepatic arteries are carefully dissected and preserved. An accessory right hepatic artery requires reconstruction prior to transplantation. Frequently, an accessory left hepatic artery cannot be safely reconstructed, because of small size, and is dissected and preserved in situ.
The recipient hepatectomy is performed in one of two ways: (1) conventional (standard bicaval) or (2) the piggyback or “cava-preserving” technique (Fig. 30.2A and B). Both approaches to the hepatectomy require initial takedown of the falciform and gastrohepatic ligaments, followed by dissection of the hilum of the liver with transaction of the hepatic artery, common bile duct, and portal vein. The conventional approach then proceeds with clamping of the vena cava above and below the liver with transaction of the vena cava between the clamps and removal of the liver. This technique necessarily requires complete clamping of the vena cava for a prolonged period of time. The piggyback technique varies from this approach with no clamping of the vena cava required. For the piggyback hepatectomy, the liver is carefully retracted away from the vena cava with perforating branches between the vena cava and the liver individually ligated and transected. Eventually, the liver remains attached only by the hepatic veins. The veins are clamped and transected, and the liver is removed. The piggyback technique is technically more difficult than the conventional approach, but the recipient tends to remain more hemodynamically stable as the preload to the heart from the lower body is never interrupted. Some surgeons who use the piggyback approach construct a temporary portacaval shunt to decompress the portomesenteric system until the time of liver allograft reperfusion. The piggyback approach may be the preferred method in high-risk patients such as the elderly, those with poor physiologic reserve, or patients who are hemodynamically unstable. The piggyback technique tends to preserve hemodynamic and physiologic stability throughout the transplant, which may then be associated with less perioperative morbidity and mortality.
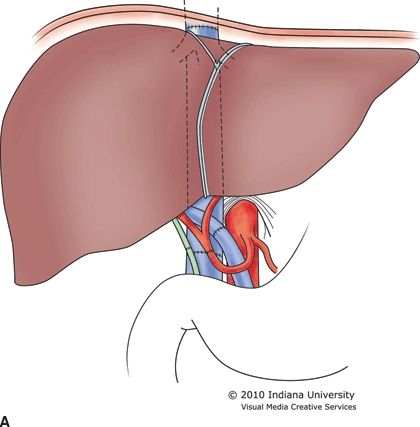
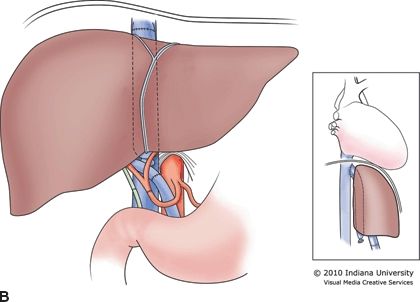
FIGURE 30.2 A. Conventional or standard bicaval anastomosis of the vena cava. B. Piggyback or “cava-preserving” technique for LT.
Finally, the transplant is performed. For the conventional approach, the vena cava must be reanastomosed both above and below the liver. For the piggyback technique, the liver outflow is through the clamped hepatic veins so that a single anastomosis is required. This reduction from two to one vena cava anastomosis can decrease critical warm ischemia time by as much as 5 to 10 minutes. Next, the portal vein and hepatic artery are anastomosed. Finally, the common bile duct can be anastomosed, after the liver has been fully reperfused, either to the recipient common bile duct or to a Roux-en-Y limb of the small intestine. At our center, 90% of our transplants employ a primary duct-to-duct reconstruction, and we never use a T tube.
In countries with limited access to deceased donors, partial liver allografts from living donors may be the only option available for patients in need of transplantation. Also, splitting of a whole deceased donor liver can result in two viable grafts, though each split portion carries an increased risk of complications when compared to a whole organ allograft. The living donor partial liver transplant requires use of the piggyback hepatectomy because there is no vena cava available from the living donor. Therefore, the donor hepatic vein is anastomosed to the recipient hepatic veins, with portal and arterial inflow constructed directly to the native portal vein and hepatic artery (Fig. 30.3). The bile duct is transected close to the liver graft and is generally reconstructed with a Roux-en-Y limb of jejunum.
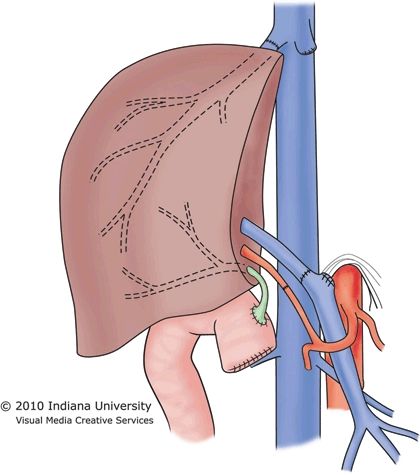
FIGURE 30.3 Implantation of the right lobe graft into an adult.
COMPLICATIONS
Immediate posttransplant complications may result from technical issues related to the transplant procedure, poor function of the graft, or recipient health issues. The most critical early posttransplant complication is thrombosis of the hepatic artery or the portal vein. Thrombosis of the hepatic vein anastomosis is very uncommon. The liver requires both hepatic artery and portal vein inflow for survival, and thrombosis of either anastomosis places the graft at imminent risk of failure. Doppler ultrasound is utilized post-LT to assure that vascular flow remains adequate. A loss of flow in either vessel generally necessitates emergent return to the operating room to reestablish flow. Posttransplant bleeding in the first 24 to 48 hours is not uncommon and requires reexploration to identify and control the source. Primary nonfunction (PNF) is a definition of exclusion in which there is graft failure within 7 days of the transplant with no other identifiable cause. PNF occurs in 1% to 5% of liver transplants and may be related to prolonged ischemia time, old donor age, and severe steatosis. Small for size graft is a syndrome seen almost exclusively in partial LT. The small size of the transplant graft in relation to the recipient blood flow results in persistent portal hypertension and liver congestion as the portomesenteric flow is too great for the partial liver graft segment. This syndrome is a major cause of early graft loss in living donor transplantation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree