FIGURE 14.1 Algorithm for investigation of small nodules found on screening in patients at risk for HCC. Adapted from AASLD practice guidelines 2010. Bruix et al. 2010, with permission.
STAGING SYSTEMS FOR HCC
HCC arises in the background of a preneoplastic cirrhotic liver, and both HCC and the underlying cirrhosis along with the patient’s condition may affect the mortality of the patient. Similarly, the biology of the disease and the risk factors are different in the East and West. HCC is the one of the few tumors that can be treated by both resection and transplantation. Because of these unique challenges, it is difficult to devise a uniform staging system and, consequently, several staging systems for HCC exist. An ideal staging system should take into account the tumor burden, functional state of the liver, and performance status of the patient. The Barcelona Clinic Liver Cancer (BCLC) staging system is currently the most widely utilized staging system worldwide as it includes tumor stage, liver functional status, and physical status of the patients and links the various stages to treatment modalities and estimated life expectancies. Figure 14.2 summarizes the BCLC staging system (Fig. 14.2).
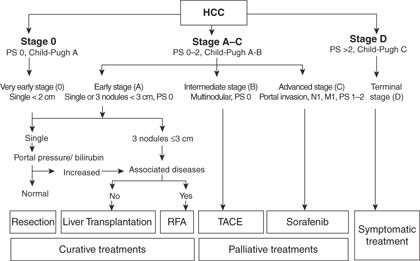
FIGURE 14.2 Barcelona Clinic Liver Cancer (BCLC) staging system. PS, performance status; N, nodal status; M, metastases. Adapted from AASLD practice guidelines 2010. Bruix et al. 2010, with permission.
TREATMENT OF HCC
There are several treatment options available to patients diagnosed with HCC. The treatment algorithms are guided by the extent of tumor burden (extrahepatic spread, vascular invasion, number of tumors, tumor diameter), severity of underlying liver dysfunction, size of future liver remnant (FLR), functional status, and comorbidities of the patient.
Transplantation and resection continue to be the major curative-intent therapeutic options available to patients with HCC. Patients with early-stage disease (i.e., HCC falling within the Milan criteria—solitary lesion ≤ 5 cm or ≤ 3 lesions with the largest diameter ≤ 3 cm and absence of macroscopic vascular invasion or extrahepatic disease) and advanced cirrhosis including Child-Pugh class B/C and portal hypertension are thought to be candidates for transplantation, whereas resection remains the treatment of choice for patients without any significant underlying liver disease. Ablative treatment, chemoembolization, and systemic therapies play an important role as well.
Liver Resection
Liver resection may be considered the primary treatment modality in some patients with HCC in the absence of cirrhosis. Patients with well-compensated cirrhosis, that is, Child-Pugh A cirrhosis, absence of portal hypertension, and Model for End-Stage Liver Disease (MELD) score of less than 10, may be considered for resection as well. Resection is usually contraindicated in patients with advanced liver disease, that is, Child-Pugh C and majority of patients with Child-Pugh B with portal hypertension.
Liver resection provides specimen for pathologic confirmation of diagnosis and can also help to ascertain other pathologic tumor factors, which may help provide information on the biology of tumor. Unlike orthotopic liver transplant (OLT), liver resection is not usually limited by tumor size/number or macrovascular invasion, and there is no waiting time. Unfortunately, the recurrence rates tend to be higher as resection does not address the premalignant potential of the FLR.
Preoperative Assessment of Liver Function
Careful preoperative assessment of liver function is important since postoperative liver failure in patients undergoing liver resection is associated with high mortality and morbidity. A complete metabolic panel is one of the most basic tests that can be performed to assess the liver function. Candidates who have evidence of active hepatitis as indicated by elevated bilirubin, AST, or ALT may be poor candidates for resection. In the Western world, the Child-Pugh scoring system has been used traditionally to estimate the hepatic functional reserve prior to liver resection. Patients with Child-Pugh class A and those with highly select Child-Pugh B are considered candidates for resection. More recently, the MELD score has been applied to preoperative selection of HCC resection candidates, as well.
Portal hypertension is considered to be present when hepatic venous pressure gradient is greater than 10 mm Hg. Portal hypertension increases the risk of major postoperative complications in the form of variceal bleeding, endotoxemia, and postoperative hepatic decompensation. Therefore, clinical or radiologic criteria to diagnose portal hypertension are a key step in the evaluation of candidates for resection. Platelet count of less than 100,000/μL associated with splenomegaly, ascites requiring drug treatment or intervention, esophagogastric varices on upper endoscopy, and presence of abdominal wall collaterals may point toward significant portal hypertension.
In the East, further functional assessment of the liver is performed preoperatively most commonly using indocyanine green (ICG) clearance. Functionally, ICG is taken up by hepatocytes and excreted in the bile in an adenosine triphosphate-dependent fashion. Therefore, its clearance from the systemic circulation is an indicator of hepatic function. The amount of ICG remaining in the bloodstream of a patient with normal liver function 15 minutes after the injection should be less than 10%. The ICG clearance test is not commonly used in the United States.
Evaluation of Future Liver Remnant Volume
Major or extended hepatectomy may lead to an inadequate FLR that can be associated with significant risk of hepatic insufficiency and subsequent mortality and morbidity. Although the risk of hepatic insufficiency is determined by several factors as highlighted above, the size of FLR continues to be one of the major determinants of postoperative hepatic failure. Precise measurements of hepatic volume are needed before operating on any patient that is likely to be left with an inadequate FLR. The FLR can be calculated using three-dimensional (3D) CT volumetry. FLR has been used as a surrogate to predict postoperative outcomes. In patients with normal liver function, FLR of at least 20% is recommended. For patients with cirrhosis and those treated with systemic chemotherapy, a higher FLR is recommended (40% for cirrhosis, 30% after systemic chemotherapy) due to the underlying liver dysfunction.
Portal Vein Embolization
A small FLR may increase the risk of posthepatectomy liver failure (PHLF). However, this can be avoided by inducing ipsilateral atrophy of the tumor-bearing liver and compensatory hypertrophy of the FLR by selectively occluding the blood flow to the tumor-bearing part of the liver. Hypertrophy of the nonembolized, non–tumor-bearing liver could be secondary to increased portal blood flow through redistribution as well as release of cytokines such as hepatocyte growth factor and transforming growth factor α and β. In general, portal vein embolization (PVE) is offered to patients with (1) normal liver function and an FLR of 25% to 30% and (2) with compromised liver function such as postchemotherapy liver damage, cirrhosis/fibrosis, cholestasis, and FLR of 35% to 40%. In the post-PVE period, the FLR undergoes rapid hypertrophy in the ensuing 3 to 4 weeks. In patients who have diabetes or cirrhosis, the hypertrophy may be delayed, and an additional 3 to 4 weeks may be required to assess the complete response. A small percentage of patients undergoing PVE may develop severe complications in the form of severe cholangitis, large abscesses, sepsis, portal venous, or mesentericoportal venous thrombosis, precluding liver resection. In a different strategy, PVE embolization can be utilized as a “stress test” for the liver. Patients who undergo sufficient hypertrophy may do well with resection, whereas those with insufficient hypertrophy are more likely to experience complications and posthepatectomy liver failure. Similarly, any disease progression seen during the period of PVE may indicate aggressive tumor biology, and resection may not change the course of the disease in these patients.
Outcomes
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree