OVERVIEW OF CHILDHOOD CANCER
There are approximately 12,400 children younger than 16 years diagnosed with cancer each year in the United States. As recently as the 1970s, a diagnosis of childhood cancer was considered uniformly fatal. Today, there is an overall 80% survival rate across the major 12 diagnostic categories of childhood cancer, and many malignancies have survival rates with a 90% chance of cure.
Despite the low incidence rates of pediatric malignancies and because of the increasing survivability of childhood cancer, there is a growing population of children in active treatment, as well as increasing number of childhood cancer survivors. These growing populations result from better supportive care and the use of aggressive, multimodal treatment strategies of chemotherapy, radiation, and surgery. Hence, there are periods of discomfort, side effects, and anxiety resulting in greater pain for pediatric cancer patients during their active cancer treatment and into survivorship. However, with careful planning, an effective pain management regimen can be developed and implemented.
The result of the aggressive treatment regimens for pediatric cancer patients is a success story. However, despite these advances in 5-year survival rates, there continues to be a high risk for the development of late effects (chronic health impairment) months and years after cancer treatment is completed. Complications of treatment include the risk for second malignant neoplasms, cardiac and/or pulmonary dysfunction, endocrinopathies, and neurocognitive deficits.
1 Indeed, there is a nearly 75% risk of childhood cancer patients having a chronic health problem by 30 years postdiagnosis.
2 Another important childhood cancer statistic that has implications for the field of pain management is the current estimated prevalence rate of childhood cancer survivors, now more than 300,000 in the United States.
3 Given this rapidly growing population of childhood cancer survivors, it is important to be familiar with the unique challenges faced both during and after completion of treatment of cancer during childhood because the increasing population of survivors has unique pain management issues, as this chapter will describe.
WHAT IS PAIN?
The International Association for the Study of Pain (IASP) defines pain as an unpleasant sensory and emotional experience associated with actual or potential tissue damage or described in terms of such damage. This definition is based on an individual’s verbal report of pain. The inability to express pain with words (e.g., for infants and some children and even adults) does not impact the sensation of noxious sensations or suffering. We learn to “read” pain or the distress associated with pain from behavioral cues (e.g., crying) and/or physiologic cues (e.g., tachycardia, systolic hypertension, sweating). With functional magnetic resonance imaging (fMRI), structural MRI, and further advances in neuroscience, we now know that there are many neuroelectrical and neurochemical changes associated with pain. And with ongoing pain, there can develop pathologic neural pain pathways and even gene changes. For example, in chronic pain, there can be changes in connectivity between various cortical areas of the brain (e.g., anterior cingulate) and the prefrontal cortex. Therefore, we now know that all experienced pain is accompanied by neural signaling changes and we can no longer ask the question “Is the pain psychological or physical?” At the same time, we also know that pain can be enhanced or diminished by emotional and cognitive factors as well.
This section will describe the different categories of pain and how to assess pain in infants and children. Nociception is the neural transmission and central of noxious stimuli, tissue injury, or inflammation, and is an important biologic function that sets up the alert system for the mechanism associated with pain sensation. On the other hand, “pain” is a conscious experience and requires the interpretation of noxious neural signals activating and connecting with various parts of the cerebral cortex. When one is unconscious, under general anesthesia, or in a deep-sleep state, presumably an individual cannot feel pain, even though various pathways of nociception are active. The experience of acute pain, with rapid transmission to pain perception areas in the brain, alerts an individual to potential or ongoing injury and prompts the avoidance or limitation of further injury. Dysfunctional acute pain inhibitory mechanisms can lead to normal protective mechanisms such as not putting one’s hand in a fire. However, such dysfunction can also lead to a variety of medical complications, including limb injury or decubitus ulcers. Acute noxious stimulation that is not under a child’s control, such as that associated with medical procedures, does not allow for the usual self-protective behavior of avoidance of the painful stimuli. Thus, we need to provide methods to reduce acute pain in these iatrogenic situations unique to the cancer care experience. Additionally, there are other avenues for ongoing nociception (e.g., cancer invasion of bones, invasion of other organs, causing intestinal obstruction) that convey no protective significance and require treatment.
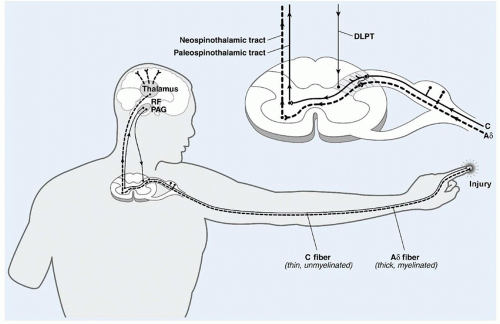
Nociceptors in deep and superficial tissues can be activated by chemical, thermal, or mechanical stimuli to send afferent impulses through myelinated Aδ fibers or unmyelinated C fibers. Between the initiation of tissue damage in the periphery and the perception of pain that follows in the brain, there occurs a complex series of physiologic events. Nociception can thus be divided into four processes: transduction, transmission, modulation, and perception.
Transduction refers to the process in which noxious stimuli are translated into electrical signals at the sensory nerve endings and transmitted to the spinal cord via Aδ and C fibers (
Fig. 42.1).
Aδ fibers are myelinated, rapidly conducting fibers (10 to 40 m/s), ending in specialized nerve endings (high-threshold mechanoreceptors). These fibers are primarily responsible for sensations of well-localized, sharp, stabbing pain, otherwise known as first pain. Aδ fibers have a high threshold for firing in response to mechanical-thermal stimuli, but once activated, they markedly increase their firing rate as the stimulus intensity increases.
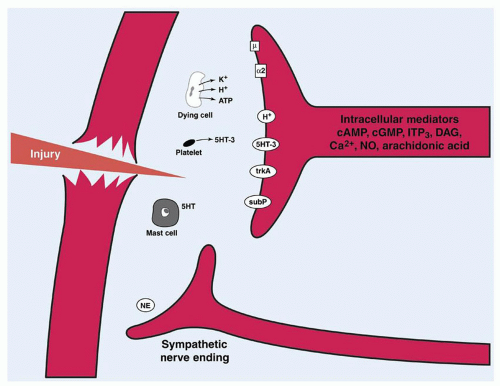
C-polymodal fibers are unmyelinated fibers with free nerve endings. They respond to noxious mechanical, thermal, and chemical stimuli at a much slower rate (<2 m/s), leading to pain characterized as dull, aching, burning, and poorly localized. This pain is known as second pain because it is usually perceived after the first pain sensation. Substances that mediate and intensify the stimulation of these nerve endings include bradykinin, prostaglandins, leukotrienes, substance P, acetylcholine, histamine, hydrogen ions, and potassium.
Sensitization is the process by which activation of neural impulses occurs at a lower-than-normal threshold of activation. Both Aδ and C-polymodal nociceptors are capable of sensitization. C-polymodal nociceptors may also widen their receptive fields and are capable of prolonged discharge relative to the stimulus duration.
In addition to the aforementioned mediators, the sympathetic nervous system may itself be sensitized and may activate primary afferent neurons (
Fig. 42.2). This may involve norepinephrine (noradrenaline) and other substances acting at α2-adrenoreceptors on the afferent neurons. This phenomenon is certainly present in
the early stages of the neuropathic pain syndromes but may also play a role in acute tissue trauma in the absence of nerve injury.
Transmission refers to propagation of the impulse through the sensory nervous system via primary afferent fibers, which synapse in the dorsal horn of the spinal cord, second-order neurons in the lamina of the dorsal horn, and ascending neurons projecting to brain stem, thalamus, and thalamocortical projections (
Fig. 42.1).
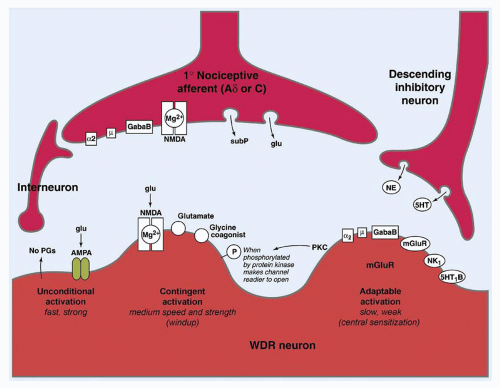
Modulation denotes the alteration of nociceptive information by endogenous mechanisms. This modulation may result in either attenuation or amplification of the initial signal. Perhaps, the most important of these sites is the dorsal horn of the spinal cord. Modulation occurs between neurons, as well as via pathways of descending inhibition originating in the thalamus and brain stem. Neurons within these pathways release inhibitory neurotransmitters, including norepinephrine, serotonin, γ-aminobutyric acid, glycine, enkephalin, and other endogenous opioids that block the release of substance P, glutamate, and other excitatory neurotransmitters (
Fig. 42.3).
Finally, perception reflects the impact of the nociceptive information on the existing cognitive-psychological framework. Perception is the emotional and cognitive experience of physical pain. That experience then changes the framework itself and thereby affects subsequent painful experiences. The term unpleasant, within the context of the IASP definition of pain, commonly means painful or painfulness in a broad sense. In contrast to the sensory dimension, it generally refers to the affective dimension of pain. This aspect of pain produces suffering, which is described as an individual’s affective experience of unpleasantness, and aversion associated with harm, real or perceived, or the threat of harm. It is this dimension, suffering, that mandates the effective treatment of pain from the humanitarian perspective.
Nociception also produces a physiologic and endocrine response separate from its conscious perception and subsequent cognitive and emotional behavioral responses. These biologic responses are measurable, are sometimes useful for quantifying pain in pre- or nonverbal patients (particularly in the research setting), and are generally medically adverse in their physiologic consequences. These adverse effects include tachycardia and hypertension, activation of glucose counterregulatory hormones, muscle spasms, and an overall increase in metabolic demands as a consequence of these effects. This dimension of pain mandates effective treatment from the medical and medico-economic perspectives. While pain is generally associated with a process of tissue injury, or potential injury, clinicians and researchers have long observed a lack of correlation between the extent of tissue injury and the intensity of pain or suffering. The experience of pain is therefore and obviously quite subjective and, as such, is modulated by developmental, familial, situational, emotional, cognitive, historical, and other factors. Assessment of the extent to which one or more of these modulating factors requires specific intervention is critical to the effective treatment of pain.
PAIN IN CHILDREN: HOW DOES IT DIFFER FROM THAT IN ADULTS?
Before a discussion can exist about the differences of pain in adults and children, it is useful to define pain within the context of childhood. The Cartesian model for conceptualizing medical conditions in general, and pain specifically, the mind-body duality, has long been abandoned in favor of the current thinking of pain as a comprehensive phenomenon consisting not only of a physical domain but also of intimately intertwined psychological and social domains; this construct is termed the
biopsychosocial model. As is well known to the pediatric oncologist, childhood is a period in which there are complex and rapid neurodevelopmental changes occurring from birth to young adulthood. In children, the need for integration of the developmental level of the child into the biopsychosocial model of pain makes pain assessment and treatment even more complex than that in adults. Children pass through five developmental stages: (1) infancy, (2) toddlerhood, (3) a preschool period, (4) a school-age period, and (5) adolescence; the relevance of these periods is projected not only to psychological effects of pain and its treatment but also to the pharmacokinetics and pharmacodynamics
of pain therapy and to anatomic and physiologic considerations in performing nerve blocks or other interventions to manage pain. These levels of development are also important because they directly affect the assessment of pain in children.
Newborns-Infants
It was previously believed that newborns and infants could not experience pain because they possessed immature neurologic systems and that only as a child developed could pain be experienced. However, ongoing basic research has dispelled this myth and proven that newborns and infants possess all the anatomic and neurologic structures needed for nociception to occur from the time of birth, do indeed respond to nociceptive stimuli, and mount a hormonal stress response to noxious stimuli that may even exceed that seen in adults. In fact, the partial development of the immature nervous system preserves nociception but may paradoxically amplify pain because the descending pain inhibitory system is the one neuroanatomical feature of pain physiology that is not fully mature at birth; therefore, a painful stimulus transmitted through the neural afferent system to the newborn brain may prove to be
more painful to the newborn than it would be to the older child or adult.
4
Infants-Preschool
From birth through early childhood, there is complete maturation of the neuroanatomy and physiology of nociception; the principal differentiating factor that distinguishes a child at this age from older peers is in the domain of intellect, psychology, and personality. A normal developmental assessment evaluates five main areas: gross motor skills, fine motor skills, language skills, personal/social skills, and cognitive skills. Changes occurring in these areas, in turn, affect the pain assessment and emotional response of the child to the painful stimuli. For example, the language skills of a 2-year-old include a 50-word vocabulary and the ability to construct two-word sentences. During this period, the child is not able to effectively describe the pain sensation and is unable to quantify it, and if inadequate pain treatment occurs as a consequence, there can be more fear and anxiety with each subsequent painful procedure. One year later, at 3 years of age, there is an expected 250-word vocabulary, 3-word sentences, and speech that is intelligible to strangers 75% of the time. These children may be more able to effectively communicate with parents and doctors and have treatment of the pain anticipated and initiated more promptly, a factor related to these enhanced communication skills that can serve to decrease the anxiety for future procedures.
School-age-Adolescence
School-aged children will have a progressively increasing ability to communicate effectively with the health care team. In turn, the providers must clearly communicate with these children about their treatment plan to minimize the anxiety children can experience from medical interventions. During adolescence, normal developmental increasing desire for autonomy necessitates direct communication by the health care team, not only with parents but also directly with the adolescent, based on the adolescent’s desire for independent decision making (when possible). However, in all levels of normal development, the impact of illness can cause pediatric patients to regress and become more dependent on parents for support of physical and emotional needs, with parents often providing the primary input to the health care team about children’s pain and effectiveness of pain management. When possible, it is always helpful for the health care team to also attempt to get the child’s and adolescent’s self-report of pain.
PAIN IN CHILDREN: CAN IT BE QUANTITATED?
Treating pain or other symptoms without attempting to quantify them in some way makes as much sense as treating blood pressure without measuring it. Historically, assessment of the quantity of pain was limited to casual and subjective observations of patients and then extrapolating these subjective observations to a treatment plan. There are at least three obvious weaknesses inherent in this approach; first, these casual observations were in fact a determination of the external manifestations of suffering, which may or may not have been proportional to the degree of pain. Second, these external manifestations of suffering and distress are affected by other internal and external factors such as amplification by fear or anxiety or reduction by nonanalgesic sedative medication. Finally, external observations are filtered through the previous experiences and biases of the observer. The science of pain measurement is imperfect and evolving; nevertheless, it is important to attempt to quantify pain in all circumstances.
In the final analysis, pain is a subjective experience known only to the patient, whereas to the clinician it is a secondhand experience. Therefore, the most reliable measure of pain is self-report, but because children vary widely in their developmental and intellectual capabilities to express pain in a quantifiable manner, several tools have been developed that are age and development specific. Although many symptom assessment instruments have been developed and validated for adults in a variety of clinical settings, there are few symptom assessment tools for children with cancer. The validated symptom assessment tools in pediatrics have focused on three common symptoms: pain, nausea, and fatigue. In contrast to instruments measuring nausea and vomiting, instruments measuring pain in children have largely been validated in predominantly the noncancer setting. These are summarized in
Table 42.1.
In addition to making an attempt in order to quantify pain, determining its qualitative characteristics will provide the clinician important clinical clues regarding both its etiology and appropriate treatment. For example, abdominal pain that is central and colicky in nature suggests a bowel obstruction or dysmotility, whereas abdominal pain that is constant, localized to a quadrant, and radiates to the back is more suggestive of solid organ pathology. Similarly, limb pain that is burning, lancinating, shooting, and associated with skin sensitivity suggests a neuropathic source of pain. This will be discussed in further detail in sections of this chapter to follow.
Newborns and Infants
Measurement of pain in newborns and infants obviously relies upon observational scales, which may be unidimensional, that is, measuring several features of behavior alone, or multidimensional, combining observations with physiologic measurements such as blood pressure and heart rate. Investigators have devised a range of observational behavioral distress scales for infants and young children, mostly emphasizing the patients’ facial expressions, crying, and body movement. Facial expression measures appear most useful and specific in neonates. The addition of measurements of autonomic and vital signs can indicate pain, but because they are nonspecific, they may reflect other processes, including fever, hypoxemia, and cardiac or renal dysfunction, that may introduce artifact into the measurement of pain.
Older Children
Children 3 to 7 years old become increasingly articulate in describing the intensity, location, and quality of pain. For the child unable to quantify pain independently, there are valid observational scales. Von Baeyer and Spagrud
5 have completed a systematic review of observational measures of pain used for children between 3 years and 18 years of age and have included the level of scientific evidence to support their use. In this review, they have identified two scales for assessing pain intensity associated with medical procedures, the Face, Legs, Arms, Cry, Consolability (FLACC) scale and the Children’s Hospital of Eastern Ontario
Pain Scale (CHEOPS).
6,7 Both these scales evaluate pain in the non- or preverbal child, and use scaled observations of posture, movement and facial expression. The FLACC rates five variables: Facial expression, Leg position, Activity, Cry, and Consolability. The minimum score is 0 and the maximum 10, similar to other commonly used pain scales. The FLACC has been well validated not only in the initially studied population of ages 1 month to 7 years, but has also shown to have validity in nonverbal children to the age of 18 years. The CHEOPS uses six variables: Cry, Facial expression, Verbalization, Torso position and movement, Touching behaviors, and Leg position. The minimum score is 4 and the maximum 13. Generally, the FLACC is easier and more intuitive to administer, and yields a pain score that is more easily interpreted by clinicians because it spans the same numerical range, zero to ten, as other commonly used scales, such as the self-report or Likert scale, the visual analog scale (VAS), etc.
Self-report measures for children include the use of drawings, pictures of faces (e.g., the Faces Pain Scale – Revised), or graded color intensities. Children 8 years and older can usually use verbal or VAS accurately.
8Verbal numerical ratings are now preferred and considered the gold standard; studies show valid and reliable ratings from children 8 years and older. The Numerical Rating Scale (NRS), or Likert scale, consists of numbers from 0 to 10, where 0 represents
no pain and 10 represents
very severe pain. (There is debate about the label for the anchor expression for the highest pain rating, but the current agreement is
not to use the “worst pain possible” since children can often imagine an even greater pain.) Note that some children may indicate that their pain is “off the scale” and give it a rating of “15” or greater on a 0 to 10 rating scale. Von Baeyer et al.
9 have also reviewed the use of body maps as a self-report of pain location and severity. The NRS and the VAS are well validated in
older children and teens, as in adults; generally the NRS is easier for clinicians to administer and for patients to report, because it does not involve drawing, paper and pen, and subsequent measurement of the result.
Cognitively Impaired Children
Measuring pain in cognitively impaired children remains a challenge; investigators are studying methods of assessing pain in this group.
10,11 Understanding pain expression and experience in this population is important because behaviors may be misinterpreted as indicating that cognitively impaired children are more insensitive to pain than are cognitively competent children. Children with trisomy 21 may express pain less precisely and more slowly than the general population. Pain in children with autism spectrum disorders may be difficult to assess because they may appear both hyposensitive and hypersensitive to many different types of sensory stimuli and they may have limited communication abilities. Some repetitive and self-injurious behaviors (e.g., head banging) might represent the limited ways in which these children have to self-regulate and soothe themselves in the face of pain (e.g., headaches). While self-reports of pain can be elicited from some children who are cognitively impaired, observational measures have better research validation across children. The Noncommunicating Child’s Pain Checklist—Postoperative Version is recommended for children aged up to 18 years. In addition, maladaptive behavior and reduction in functions may also indicate pain. Studies have shown that children with severe cognitive impairments experience pain frequently, mostly not because of accidental injury. Children with the fewest abilities to communicate often experience the most pain.
Measurement of Pain in Research and Drug Development
Although many pain assessment scales exist for use in infants and children, relatively few have been rigorously, statistically validated to be used in research and therapeutic drug development. When selecting a tool for pain measurement, confirm that (1) the tool has been validated in the relevant age group and (2) the tool has validity for the model of pain (e.g., chronic pain, acute surgical pain, neuropathic pain) under investigation. It is common to see a pain scale validated in one clinical circumstance applied to a different pain model without justification or statistical validity.
Table 42.1 lists the statistically validated pain scales and the clinical circumstances in which they have been validated.
12,13,14
TREATMENT OF PAIN IN PEDIATRIC CANCER PATIENTS
There are seven main categories of pain in children with cancer: (1) pain associated with medical procedures used for cancer diagnosis and treatment; (2) pain associated with bone marrow infiltration by malignant disease; (3) neuropathic pain and pain due to central nervous system (CNS) tumors; (4) bone pain and amputation pain; (5) visceral pain; (6) pain associated with the complications of bone marrow transplantation (BMT), such as oral mucositis; and (7) stress, anxiety, and other symptoms.
Pain Due to Bone Marrow Infiltration with Cancer
Acute leukemias, ALL, and acute myelogenous leukemia (AML) are the most common forms of pediatric cancer.
30 The rapid growth of leukemic blasts within the bone marrow commonly results in the experience of diffuse bone pain. The clinical presentation of the bone pain, however, is variable depending on the age of the patient. Very young children, such as toddlers, who are most commonly affected by ALL, may present with a limp or inability to walk. A school-aged child who is able to provide a pain narrative may report diffuse total body pain that is poorly localized. An adolescent patient may have back pain that he or she associates with a sports injury and may localize the pain to a specific area in a long bone. There are also solid tumors, such as neuroblastoma, which most commonly present during the toddler years and which can metastasize to either bone marrow or bone and also present as a limp or as localized pain to a specific bony area.
The most effective method of eradication of pain due to bone marrow infiltration begins with treatment of the primary disease. For ALL, induction chemotherapy regimens generally place children into remission within a 1-month treatment course.
31 For most standard risks ALL patients, by day 7 of treatment, there is a significant reduction in the leukemic burden to less than 25% of the bone marrow being affected. This decrease in leukemic burden, in turn, results in marked improvement in the bone pain. Adolescents with acute leukemias, however, often have more resistant disease and may have longer periods of acute pain due to the persistence of blasts within the bone marrow and may require analgesics throughout the induction chemotherapy regimen.
32 For patients with bone metastases from solid tumors, such as neuroblastoma, effective treatment may also include local radiation therapy to the affected site.
Pharmacologic regimens for the treatment of pain from bone marrow infiltration with cancer for both children and adolescents include the use of parenteral opioids, such as morphine or hydromorphone. Opioid analgesics may be administered through bolus dosing regimens if the pain is not constant. However, for patients experiencing continuous pain, the delivery of the opioid should include the use of a patient-controlled analgesia (PCA) component.
Often, health care providers use adjuvant pain medications, such as acetaminophen (N-acetyl-p-aminophenol [APAP]) or nonsteroidal anti-inflammatory drugs (NSAIDs), together with opioid analgesics, to optimize pain control and minimize the side effects of opioids such as respiratory depression at high doses.
33 However, in the patient with bone marrow infiltration, there is frequently thrombocytopenia. As a result, the use of NSAIDs is not recommended for these patients because of the antiplatelet effects of this class of analgesics that may place the pediatric cancer patient at increased risk for hemorrhage secondary to both the quantitative and qualitative platelet defects. Analgesia with an oral coxib, celecoxib, is an excellent alternative in this circumstance (see the following text).
Neuropathic Pain
Pediatric cancer patients can experience neuropathic pain for a variety of reasons including nerve invasion and inflammation of a nerve root due to the malignancy or infectious process, or from cancer treatment side effects. These etiologies include (1) chemotherapy-related peripheral neuropathies, such as with vincristine or vinblastine neurotoxicity
34; (2) neural compression or invasion by tumors, such as pelvic sarcomas; and (3) infectious etiologies, such as postherpetic neuralgia and neuropathies secondary to herpes zoster reactivation associated with immunosuppression. In addition, direct injury to or compression of nerve or spinal cord can occur with soft tissue sarcomas arising in the pelvis, such as Ewing sarcoma or rhabdomyosarcoma; such a patient can present with complaints of abdominal pain, lower extremity weakness,
and/or bladder and bowel dysfunction from involvement of the sacral nerves. The medications that are most effective for the management of neuropathic pain are not the opioids but rather the tricyclic antidepressants (TCAs), such as amitriptyline and nortriptyline, the newer selective serotonin norepinephrine reuptake inhibitors (SSNRIs), such as venlaxefine and duloxetine, the antiepileptic drugs (AEDs), such as gabapentin and pregabalin, and local anesthetics including the topical application of 5% lidocaine patch or intravenous administration of lidocaine.
35,36,37,38 In addition, a recent Cochrane review of the adult pain literature recommended that future research on the treatment of neuropathic pain include the assessment of effectiveness for other naturally occurring antidepressants such as St Johns wort and L-tryptophan since there are currently insufficient data to support their use or disuse.
39 Finally, as further basic research leads to greater understanding of the mechanisms of genetic and acquired neuropathic pain states, there is promise that in the near future there will be disease modifying treatments of severe pain including chemical manipulation of neuronal sodium channels, and spinal cord glial cell inhibitors.
Opioids, including the long-acting opioid methadone, can also be used for severe neuropathic pain that has not been effectively treated with TCAs or anticonvulsant medications, although neuropathic pain is notoriously resistant to opioid analgesia.
40 However, opioids are associated with a risk profile that has not been widely studied in children and there is a growing national concern of the risk of prescription opioid toxicity and death.
41 Thus, when considering the use of opioids for neuropathic pain, one should first maximize the total recommended daily dose of TCAs and AEDs, given their relatively low toxicity profile, before initiating a longterm opioid regimen.
Mechanical approaches are also important to consider in the treatment regimen for neuropathic pain. These include the use of physical therapy and acupuncture. Physical therapy should be considered for those patients who have neuropathic pain particularly due to vinca alkaloids, since these agents also cause motor weakness. Although physical therapy can be an effective therapeutic modality itself, there are several barriers to its utilization in cancer patients,
42 including financial and/or insurance barriers. A pediatric cancer patient may be too fatigued to engage in physical conditioning and exercises. The physical therapy regimens also require a significant time commitment by the patient and family. But with persistence, and pairing of the child to a single therapist with whom he or she can form a relationship of trust and who can then motivate the patient to participate regularly in the recommended exercise regimen, physical therapy is an effective, nonpharmacologic modality to supplement pharmacologic regimens that are insufficient per se at relieving the neuropathic pain.
Pain Associated with Central Nervous System Tumors
CNS tumors are the most common solid tumors diagnosed during childhood. Because of the mass effect of the brain tumor or the mechanical obstruction of cerebrospinal fluid (CSF) flow and secondary hydrocephalus, children usually present with symptoms of headaches due to increased intracranial pressure. To decrease the intracranial pressure and thereby treat the pain/headache of children, different therapeutic approaches can be used. For some brain tumors that are locally invasive but without metastatic potential, such as low-grade gliomas (astrocytomas), complete surgical removal is the treatment of choice. Dexamethasone is usually used in the preoperative period to relieve the cytotoxic cerebral edema that surrounds malignancies, and this is often remarkably effective in relieving the head pain associated with brain tumors even before surgical excision.
If the child with a brain tumor continues to report headaches postoperatively, NSAIDs, such as ibuprofen, can be considered 24 hours after surgery if there is no plan for bone marrow-inhibiting chemotherapy and/or radiation therapy. Celecoxib may safely be used in these circumstances. If an NSAID is used in the pain treatment regimen, a histamine H2-receptor antagonist to inhibit stomach acid production is thought to provide protection against gastritis or ulceration, particularly in the patient treated with dexamethasone.
For malignant brain tumors with metastatic potential, such as medulloblastomas, chemotherapy and radiation therapy follow the surgical treatment regimen. Not all primary resections of malignant brain tumors result in complete surgical removal of the tumor; therefore, pain may persist because of residual disease. Opioids, such as morphine sulfate, administered intravenously are the preferred pharmacologic medication of choice. When using high dose or prolonged courses of opioids for the treatment of CNS pain from brain tumors, the treatment plan must also include a bowel regimen, such as docusate or polyethylene glycol, to reduce the likelihood of constipation. This is discussed in more detail later in this chapter.
Benzodiazepines, such as midazolam and lorazepam, may be used as an adjunct for the treatment of pain due to CNS tumors, even though this class of medications has no direct analgesic effect. Its utility includes decreasing anxiety, decreasing muscle spasm that may occur postoperatively, and facilitating sleep. Postoperatively after the resection of a CNS tumor, children typically remain within the intensive care unit (ICU) for intracranial pressure monitoring or external CSF drainage; here, benzodiazepines are commonly used by continuous infusion or bolus dosing to minimize anxiety and agitation. Benzodiazepines are often used concomitantly with opioid analgesics outside the ICU when significant postoperative pain is reported, observed, or expected, or to alleviate painful cervical muscle spasm and pain following posterior fossa craniotomies.
Pain Associated with Primary Malignant Bone Tumors
Primary malignant bone tumors, osteosarcoma, and Ewing sarcoma result in significant pain, pain resulting from both destruction of normal trabecular bone from direct tumor invasion and intense tissue inflammation surrounding the malignancy. Thus, the pain can often be very severe and requires the use of opioid analgesic medications or interventional pain techniques. Because bone tumors have such an inflammatory component, the pain is remarkably responsive to NSAIDs, which should be considered with caution given the risk for bleeding in the patient receiving chemotherapy and are at risk for thrombocytopenia. Coxib nonsteroidal are alternatives that are not associated with platelet inhibition or gastric ulceration. Because most common primary malignant bone tumors usually occur during adolescence, PCA is the preferred method for the delivery of parenteral opioids giving the patient control over the dosing regimen. For continuous, longterm pain associated with bone tumors for outpatient management, long-acting oral opioids such as methadone or controlled-release oxycodone is the best alternative for long pharmacologic half-life and duration of effect.
Phantom Limb Pain
Amputation as well as limb-salvage procedures may be used to achieve local control of a bone tumor, and each in turn can result in chronic pain perceived to originate in the amputated limb. There are several etiologies for the occurrence of phantom limb pain, including spontaneous electrical activity of amputated nerve trunks and abnormal regrowth of nerve trunks (neuromas), each resulting in painful electrical discharges to the spinal cord and the brain. There may also be abnormalities in the CNS in response to the loss of limb, in which there may be loss of inhibitory sensory input from the amputated limb. Phantom limb pain is often severe and very challenging to treat. Systematic analyses of studies to effectively treat chronic postoperative phantom limb pain have
not clearly demonstrated an effective treatment option.
43,44 Central strategies, such as hypnotherapy, aimed at altering metabolic activity in pain perception areas of the brain, such as the anterior cingulate cortical area, show promise over peripheral strategies or opioids.
Because the incidence of phantom limb pain may be correlated with the incidence of presurgical pain in the diseased limb,
45 the first important principle in the treatment of phantom limb pain is to effectively treat the bone pain preoperatively before the amputation. This can include the use of regional anesthetic techniques, such as continuous epidural analgesia, with the use of opioids and local anesthetic to reduce dorsal horn sensitization prior to surgical resection. Preemptive use of nonconventional analgesics that slow the rate of spontaneous neuronal discharge, for example by the use of the AEDs, may also have a role in preventing phantom pain. Following the surgical amputation, treatment of the acute postoperative pain must be initiated promptly in attempt to minimize the development of a chronic pain syndrome. Strategies for the acute postoperative pain for amputees include continuous peripheral nerve blocks, continuous plexus blocks, or epidural blocks. Given that long-term opioid use is not usually effective for the phantom limb pain syndrome, AEDs and/or TCAs may be empirically initiated or continued from the preoperative period, though there is no literature support for this recommendation.
46,47Complementary and alternative medicine (CAM) options for the treatment of chronic pain due to phantom limb pain include the use of hypnotherapy, massage, and acupuncture.
48,49 While no randomized clinical studies provide support for using nonpharmacologic strategies to treat phantom limb pain, neuroimaging research demonstrates that specific areas of the brain show decreased arousal associated with these practices, interpreted as a reinterpretation of the chronic pain experience.
50
Visceral Pain
The four major etiologies of visceral pain include (1) organ invasion with capsular wall stretching; (2) organ compression; (3) hollow organ obstruction (e.g., ureter or bowel); and (4) or peritoneal cavity bleeding. For all etiologies of visceral abdominal pain, it is important to initiate the treatment of the pain early in an attempt to prevent visceral hyperalgesia and subsequent centralization of pain. Visceral hyperalgesia results from an insult, such as tumor invasion or surgical manipulation, to the celiac plexus (consisting of the hepatic plexus, splenic plexus, gastric plexuses, pancreatic plexus, supra/renal plexuses, testicular plexus, superior mesenteric plexus, and the inferior mesenteric plexus). If treatment of the visceral pain is not initiated promptly or effectively, ongoing nociception ultimately may result in a central pain syndrome, which can be very difficult to treat.
For visceral pain due to organ invasion with capsular wall stretching or tumor regrowth, the primary treatment to decrease the pain is to decrease the tumor size by surgery, chemotherapy, and/or radiation therapy. Of course, surgical resection of an abdominal tumor will result in significant postoperative pain; therefore, a treatment plan should be in place to manage the surgical pain to expedite postoperative recovery. The management of surgical pain with parenteral opioids, regional anesthetic infusions, adjunctive agents, and other techniques is discussed elsewhere.
51,52,53Regional nerve blocks and abdominal plexus blocks are also a consideration for the treatment of visceral pain due to tumor invasion of a solid organ or abdominal nerve plexus. They can be used in the pediatric population by experienced pediatric anesthesia or pain management consultants for the management of pain in unresponsive abdomino-pelvic tumors, such as bladder rhabdomyosarcoma or neuroblastoma.
Visceral abdominal pain can also be due to intestinal obstruction either from the tumor mass or from intestinal adhesions that are the consequence of prior abdominal surgical procedures. The treatment of the pain of a bowel obstruction is, of course, mechanical relief of the bowel distention first by nasogastric suction and then, ultimately, by surgical resection of the obstruction. Opioids are an effective measure to temporize while awaiting definitive surgical treatment and act by their analgesic properties as well as by decreasing the peristaltic activity of the gut.
Pain Associated with Stem Cell Transplantation
Pediatric cancer patients requiring stem cell transplantation (SCT) as part of their treatment regimen are considered a high-risk group of patients for the development of acute and chronic pain, given the intensity of this treatment regimen. Mechanisms of acute pain associated with BMT include mucositis, graft-versus-host disease (GVHD), and infectious complications. Thus, pain management is critical in the care of the BMT patient, and if pain is not treated adequately, it can result in decreased quality of life (QOL).
Mucositis
Although mucositis occurs when chemotherapy alone is given, mucositis is often more prolonged and severe in BMT patients given their prolonged periods of neutropenia.
54 The grading scale for describing the severity of oral mucositis established by the World Health Organization (WHO) begins with mild, or grade I mucositis, in which the oral mucosa is red and tender, to severe, grade IV mucositis, in which the pediatric cancer patient is unable to eat and maintain his or her own nutrition.
55 The severity of the mucositis often corresponds to the description of the severity of the pain by the patient. Grade I mucositis may only require intermittent bolus dosing of an opioid analgesic. In the BMT setting, however, mucositis pain usually requires use of an opioid, ideally administered by both continuous intravenous administration and PCA, given the duration of pain over a period that may last for 2 or more weeks and the extensive tissue injury involving the entire gastrointestinal tract.
56 When marrow engraftment occurs and counts recover, the PCA is usually weaned relatively quickly and discontinued however signs of withdrawal must be assessed depending on the length of time the patient has been on the PCA.
Graft-Versus-Host Disease
Acute GVHD manifested by skin rash, right upper quadrant pain, and/or diarrhea is a source of peripheral somatic and abdominal pain. The skin rash ranges from mild erythema of the palms and soles of the feet to painful bullous desquamation in the severe form. Chronic GVHD occurs 100 days beyond the hematopoietic stem cell infusion and is thought to be an autoimmune process. Chronic GVHD primarily has skin manifestations that include scleroderma-type changes often accompanied by joint stiffness and immobility.
Treatment of Pain Due to GVHD
Treatment of pain due to acute gastrointestinal GVHD, often presenting as crampy abdominal pain due to the diffuse enteritis, requires the use of an opioid analgesic in the form of a PCA both for analgesia and to reduce hypermotility. Chronic GVHD is usually an outpatient disease requiring a long-term pain management plan since the autoimmune process can cause muscle and organ invasion resulting in chronic neuropathic pain. In the setting of chronic pain, oral opioid use may be required until the neuropathic pain is adequately treated with alternative agents, including AEDs define such as gabapentin or TCAs define such as amitriptyline. Nonpharmacologic therapies, including the use of acupuncture, massage therapy, and other relaxation strategies, may also be considered. No randomized controlled analgesic trials have been published to guide the management of pain in chronic GVHD.
Other Factors Important to Pain Assessment and Development of the Treatment Plan for Stem Cell Transplantation Pediatric Patients
There often is significant anxiety experienced by the pediatric patients who have undergone SCT, due to prolonged hospitalization, frequent procedures and episodes of pain, and fear of impending death. Thus, both pharmacologic and psychosocial support services to decrease anxiety of the children are useful. Facilitation of night sleep is also valuable and may be enhanced by the pharmacologic use of psychotropic drugs, such as amitriptyline or trazodone, with sedative side effects.