Overview of Venous Thromboembolism
Sam Schulman
Victor J. Marder
Gilbert C. White II
HISTORICAL BACKGROUND
Thrombosis as a clinical condition was probably described almost 5,000 years ago, when the Chinese emperor Huang Ti wrote of a likely arterial event, “when it coagulates within the pulse the blood ceases to circulate beneficially; when the blood coagulates within the foot it causes pains and chills.”1 The Greek physician Claudius Galen (131 to 201 ad) coined the term “thrombosis,” which means curdling, but the first thorough analysis of the pathogenesis of thrombosis was described by the German pathologist Rudolf Virchow (1821 to 1902).2 He concluded that “consequences” (rather than causes) “of the obstruction could be grouped into three categories,” (a) irritation of the vessel and its surroundings, (b) blood coagulation, and (c) interruption of the blood stream,2 the combination of which was later named Virchow’s triad.
RISK FACTORS FOR VENOUS THROMBOEMBOLISM
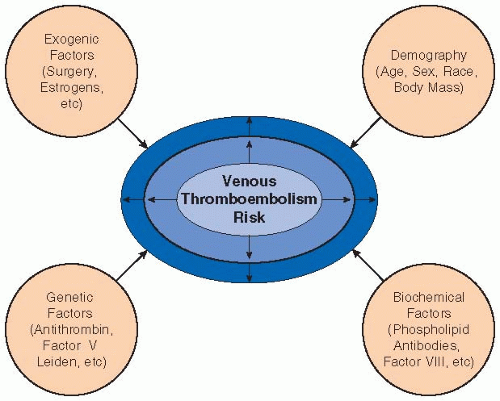
Research during the past 3 to 4 decades has identified abnormalities that are etiologic factors for venous thromboembolism (VTE).3 These abnormalities can be categorized as hereditary or acquired, or, alternatively, as increased coagulation factor activity (factor V Leiden, prothrombin G20210A mutation), decreased coagulation inhibitor levels (antithrombin, protein C, endothelial protein C receptor, protein S), or antiphospholipid antibody disruption of hemostasis. In addition, abnormalities of the fibrinolytic system and protective mutations of coagulation factors (e.g., factor XIII Val34Leu) may have roles in thrombosis. The hereditary thrombophilias (see Chapter 81) mainly contribute to VTE, but there is controversy as to their importance in arterial thrombosis.4,5 On the contrary, the antiphospholipid syndrome is associated with both venous and arterial thromboembolism (see Chapter 101).
The need for investigation of thrombophilia in patients with VTE is controversial. Screening for thrombophilia in patients without previous VTE, but who are at increased risk (estrogens, pregnancy, major surgery), is hardly ever cost-effective.6 Acquired risk factors can be temporary, chronic, or permanent. Temporary risks include surgery, immobilization, estrogen exposure, L-asparaginase treatment, long-haul travel, infection, heparin-induced thrombocytopenia, and indwelling catheters. Long-distance travel may or may not present a need for prophylactic therapy; for example, if deep-vein thrombosis (DVT) after an 8-hour car trip 1 month earlier is the only identifiable risk factor, one may be hesitant about the association and whether the thrombosis is provoked or unprovoked. Chronic or permanent risk factors include immobilization such as after spinal cord injury or stroke, inflammatory bowel disease, systemic lupus erythematosis, nephrotic syndrome, cancer, myeloproliferative disorders, and paroxysmal nocturnal hematuria (see FIGURE 80.1 for more information).
The reported incidence of VTE varies from 104 per 100,000 in a study of medical records in Worcester, Massachusetts,7 to 387 per 100,000 in a cohort study in Sweden.8 Differences relate to methodology for data capturing, selection of population, and diagnostic techniques. There is a definite need to establish trends accurately in order to show when implementation of preventive measures has had any impact and also to determine how changes of patient demographics (age, body weight) play out in the disease panorama (see Chapter 82).
DIAGNOSIS OF VENOUS THROMBOEMBOLISM
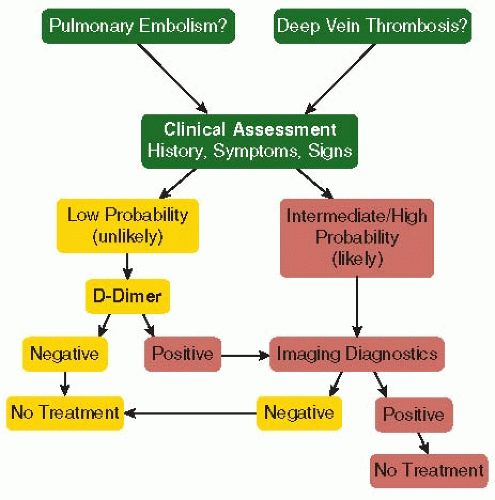
Clinical symptoms and signs of DVT or pulmonary embolism (PE) are not sufficient for diagnosis, but the combination of risk factors and select symptoms and signs is used in clinical prediction, for example, with the Wells score for DVT or PE9,10 or the Geneva score for PE.11 The revised Wells scores for DVT and PE allow one to utilize low, moderate, or high probability of VTE for assessing unlikely or likely outcomes.12,13 Patients with unlikely or low probability of VTE can be assessed with a D-dimer test, which, if negative, allows for a highly negative predictive value for VTE. This strategy allows for a 50% reduction of expensive imaging for VTE diagnosis.
The “gold standard” of imaging of the leg veins using venography has been almost entirely abandoned in favor of noninvasive compression ultrasound. Similarly, computed tomography of the pulmonary arteries (chest CT) for imaging of PE is utilized more often than ventilation-perfusion lung scanning (see Chapter 83). In spite of progress in diagnostic approach, there are unresolved issues, for example, the extent of ultrasound examination, DVT recurrence, diagnosis of PE in pregnancy, and VTE assessment of suspected PE in hospitalized patients with elevated D-dimer levels. The PIOPED III study demonstrated that gadoliniumenhanced magnetic resonance angiography has a sensitivity of 78%, which is suboptimal, and many centers have a high proportion of technically inadequate images.14 Conversely, multidetector chest CT may be too sensitive, identifying subsegmental filling defects of questionable clinical significance.
The assessment of the emergency room patient with possible VTE requires not only a correct diagnosis but also an assessment of risk. This is of particular importance for patients with PE, for whom there is no high-quality evidence for the safety of outpatient treatment. The Pulmonary Embolism Severity Score, based on identifying risk strata that allow prognostic accuracy,15,16 describes 30% of patients with low risk who have
a 30-day mortality of 1% and can be treated as outpatients. For the remaining 70% of patients, cardiac biomarkers (troponin, brain natriuretic peptide [BNP], pro-BNP, and heart-type fatty acid-binding proteins) combined with clinical score may be useful to identify additional patients for home treatment.
a 30-day mortality of 1% and can be treated as outpatients. For the remaining 70% of patients, cardiac biomarkers (troponin, brain natriuretic peptide [BNP], pro-BNP, and heart-type fatty acid-binding proteins) combined with clinical score may be useful to identify additional patients for home treatment.
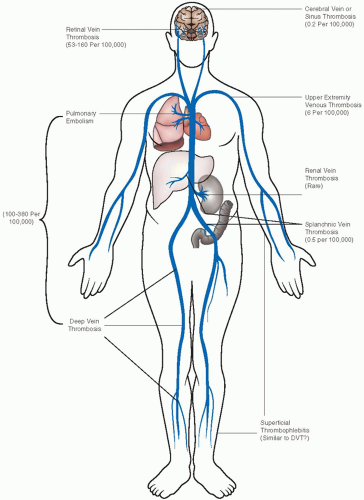
LOCATION OF VENOUS THROMBOEMBOLISM
Most DVT occurs in the lower extremity, and consequently almost all randomized controlled trials on management are based on this thrombus location. Upper extremity thrombosis of the brachial, axillary, and subclavian veins constitutes 4% to 10% of DVT,17 and chronic sequelae with venous insufficiency are not uncommon (FIGURE 80.2). The basilic and cephalic veins are superficial, not deep veins.
Superficial phlebitis is mainly an inflammatory process secondary to chemical or mechanical irritants or in varicose veins with very slow blood flow. It is often complicated by a self-limited clot formation. It is difficult to find any data on the incidence of superficial (thrombo-) phlebitis, but in an Italian study of 15,000 patients seeking advice from their general practitioner for any medical condition, there was a history of this disorder in 10.8% of the women and 4.9% of the men.18 It is unclear how patients should be selected for 4 to 6 weeks of treatment with low molecular weight heparin (LMWH) or fondaparinux versus short-term anti-inflammatory therapy.19
The most common acquired cause for upper extremity DVT is an indwelling catheter; thoracic outlet syndrome accounts for 30% of upper extremity DVT (2 events/100,000/year).
Cerebral vein or sinus thrombosis is diagnosed in two persons per million each year.20 Patients can present with headache, papilledema with blindness, seizures, hemianopsia, aphasia, hemiparesis, or cognitive disorders, depending upon thrombus location and infarct extension. Nowadays, the diagnosis is usually obtained early with MRI or CT, and appropriate treatments can be started to reduce intracranial hypertension, avoid seizures, and control the clot size (FIGURE 80.3). The prognosis has improved compared to a few decades ago; the ISCVT study of 624 patients showed 80% recovery with fatal outcome in 8%.21 Residual headache is usually related to chronic intracranial hypertension, and continuing seizure activity occurs in 10% of patients, less so after the first year.
Splanchnic vein thrombosis includes mesenteric, portal, and hepatic (Budd-Chiari syndrome) vein thrombosis. The incidence of Budd-Chiari syndrome or isolated mesenteric vein thrombosis is <1 in a million per year, whereas extrahepatic portal vein thrombosis occurs in at least 4 per million.22 The majority of mesenteric or portal vein thrombosis occurs in the presence of other disorders, mainly cancer but also chronic myeloproliferative disorders, hepatic cirrhosis, pancreatitis, inflammatory bowel disease, or as a postoperative complication. Hypercoagulability plays an important role with inherited thrombophilia identified in one-third of the patients.
Among patients with thrombosis associated with myeloproliferative disease, the JAK2 V617F mutation is highly prevalent,23 present in up to 96.5% of patients. There is no controversy regarding the need for anticoagulant therapy, but the duration of secondary prophylaxis must balance the risks of recurrence against that of bleeding.
Renal vein thrombosis is a rare complication in severely ill neonates, often with poor prognosis for the renal function.24 It also occurs in adults associated with renal transplantation, with nephrotic syndrome with urinary loss of antithrombin and possibly free protein S, or with severe thrombophilia. The condition is best diagnosed with CT angiography.25
Retinal vein occlusion (RVO) differs from the other types of unusual-site thrombosis in that it is largely a primary vascular disease, associated with systemic atherosclerotic risk factors such as hypertension, diabetes, and hyperlipidemia. RVO occurs in 5.2 per 1,000 individuals who are 30 or older26 and 2.1% over 10 years among patients with ocular hypertension.27 There is controversy regarding the importance of traditional risk factors for DVT, the highest yield provided by tests for antiphospholipid syndrome.28 A randomized study comparing LMWH with aspirin in 58 patients with RVO showed better functional longterm results with anticoagulation, but this result requires confirmation.29 Unusual sites of DVT are described in Chapter 84.
LONG-TERM CONSEQUENCES OF VENOUS THROMBOEMBOLISM
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree