Overview of Vascular Biology
William C. Aird
OVERVIEW OF VASCULAR BIOLOGY
The coagulation system has evolved to prevent excessive blood loss at sites of vascular injury. As much as the fluid phase of clotting is readily amenable to detailed molecular and biochemical dissection, thrombus formation is typically localized at the blood vessel wall. Indeed, the vascular wall plays a central role in orchestrating the hemostatic response. Thus, a full appreciation of the mechanisms of coagulation requires a sound understanding of vascular biology. In recognition of the central role of the blood vessel wall in hemostasis and owing to significant advances in this area over the past 6 years, we have expanded the section on Vascular Biology in this sixth edition of Hemostasis and Thrombosis. By way of an overview, we wish to emphasize several themes that are relevant to this section.
ANGIOGENESIS: MULTIPLE LINKS WITH HEMOSTASIS
The formation of new blood vessels is essential during embryonic development (ontogeny). Angiogenesis continues to play an important role in the early postnatal period in the lung, heart, brain, and retina1 and resumes and/or continues into adulthood with the reproductive cycle, wound healing, and post-ischemic tissue repair. Qualitative and quantitative abnormalities in angiogenesis underlie many disease states. It has long been recognized that hemostasis must be highly coordinated with angiogenesis to prevent excessive blood loss or thrombosis prior to the stabilization of newly formed vessels. Fibrin provides a provisional matrix scaffold along which endothelial cells can migrate. Also, fibrin serves as a sustained release reservoir for endothelial growth factors and fibrinolytic enzymes. Platelets release vascular endothelial growth factor (VEGF), and VEGF signaling, in turn, alters the expression of procoagulant and anticoagulant proteins in endothelial cells.
Clotting factors not only cleave downstream substrates in the clotting cascade but they also cleave and activate receptors on the surfaces of cells, including endothelial cells (see Chapter 44). The importance of these cellular effects is evidenced by studies of knockout mice. For example, deletion of tissue factor, but not fibrinogen, is embryonic lethal and is associated with abnormal vascular development.2,3 Mice that are null for the thrombin receptor, protease-activated receptor 1 (PAR-1) may survive, but they too develop abnormal vasculature.4 The latter phenotype is rescued by expression of endothelial PAR-1.5 These and other findings suggest that thrombin-PAR-1 signaling in the endothelium is important for vascular development, independent of its effect on clotting.
In addition to their role as signaling molecules, proteins of the coagulation system have been found to harbor cryptic antiangiogenic fragments. For example, Judah Folkman’s group first reported that angiostatin corresponds to the first four kringle domains of plasminogen.6
Since the last edition of Hemostasis and Thrombosis, the field of angiogenesis has witnessed significant advances. For example, as outlined in Chapter 38, we now have novel insights into the mesodermal origin of endothelial cells from different sites in the vasculature, not just arteries and veins but also capillaries and the endocardium. There is new information about the interplay between tip and stalk cells and mechanisms of lumen formation. Finally, there is an evolving awareness of the importance of lymphatics and their endothelial lining as dynamic components of the cardiovascular system.
THE BLOOD VESSEL WALL: A STRUCTURAL AND FUNCTIONAL ENTITY
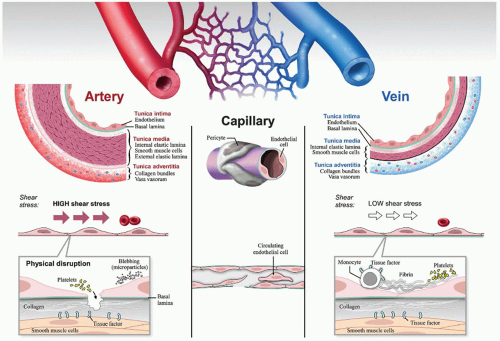
The anatomy and histology of the vascular wall have been accurately described for over two centuries (figure 37.1). As originally portrayed, the vasculature comprises a system of blood vessels aligned in series, beginning with arteries, followed by arterioles, capillaries, venules, and veins. There are some interesting exceptions to this canonical arrangement. For example, the hepatic artery and portal vein both empty into the capillaries of the liver (hepatic sinusoids). The glomerular capillaries drain into arterioles, not venules. In general, arteries have thicker walls than veins and carry oxygenated blood. However, that is not always the case. For example, the oxygen content of the pulmonary artery is less than that of the pulmonary vein. The same is true for the umbilical artery compared with umbilical vein.
Light microscopy and special stains reveal site-specific differences not only in wall thickness between different segments of the vasculature but also in the size and composition of different layers in the wall, including the intima, media, and adventitia. Electron microscopic studies have demonstrated remarkable ultrastructural heterogeneity at the level of the endothelium. Histochemical analyses have provided important information about the molecular composition of the blood vessel wall. Endothelial cells and smooth muscle cells express distinct patterns of genes, the nature of which varies between different regions of the vasculature. The subendothelial surface is rich not only in collagen but also in heparan, von Willebrand factor, and tissue factor. Indeed, presence of tissue factor on the abluminal side of the endothelium (on smooth muscle cells and fibroblasts) has given rise to the concept of a “hemostatic envelope” surrounding the vasculature.
Like other components of the vessel wall, tissue factor levels vary between segments of the vasculature.7 For example, expression is particularly high in the heart (tissue factor is expressed by cardiomyocytes), which may help explain why patients with hemophilia do not bleed into their hearts, despite the extreme forces associated with the cardiac cycle. Although tissue factor is readily expressed in cultured endothelial cells, there is little evidence that it is normally expressed in the intact endothelium.7 Aberrant expression of tissue factor in the endothelium has been reported in certain diseases states, including atherosclerosis, tumors, and sickle cell disease, where it likely contributes to the underlying pathophysiology. Tissue factor is also expressed by circulating monoyctes. The relative role of vessel wall and hematopoietic tissue factor in mediating clot formation has been the subject of considerable debate and investigation.8,9
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree