Overview of Clinical Bone Marrow Transplantation
INTRODUCTION
Bone marrow or hematopoietic cell transplantation (BMT or HCT) is a potentially curative therapy for a wide variety of life-threatening congenital and acquired hematopoietic stem cell disorders and neoplastic diseases. With the development of human leukocyte antigen (HLA) typing to identify suitably matched donors, advances in tolerability and efficacy of conditioning regimens, improvements in supportive care, and advances in the prophylaxis and treatment of graft-versus-host disease (GVHD), clinical HCT became a reality. Many of the initial clinical HCT efforts were directed toward severe aplastic anemia and acute leukemia (which remains the paradigm for allogeneic HCT in adults). However, the demonstration of lasting donor lymphohematopoietic reconstitution, the powerful cytoreductive effect of intensive pretransplantation conditioning therapy, and the exploitation of a potent immunologically-mediated graft-versus-tumor (GVT) effect led to the successful application of HCT as up-front and salvage therapy for the many hematologic malignancies and other disorders shown in Table 38-1. Some applications of HCT are potentially curative, e.g., allogeneic transplantation for acute myeloid leukemia (AML). In other settings, HCT is primarily utilized to lengthen disease-free intervals without expectation of cure, e.g., autologous transplantation for multiple myeloma. In parallel with the above advances, the use of hematopoietic cell transplantation has expanded yearly. In 2009, more than 26,000 transplants were performed worldwide, over 15,000 of these being allogeneic HCTs (1).
The principal functions of HCT are to provide:
1. Rescue (i.e., by the infusion of pluripotent hematopoietic progenitor cells in the setting of cytoreductive chemotherapy that eradicates malignant cells but also ablates stem cells and other hematopoietic bone marrow elements)
2. Replacement (i.e., of a diseased hematopoietic stem cell population by healthy stem cells capable of regeneration of multiple hematopoietic lineages)
3. An immunologic platform (As mixed lymphohematopoietic chimerism often occurs after less intensive conditioning, HCT may be envisioned as creating an immunologic platform for adoptive cellular therapy. Subsequent manipulation may occur through donor lymphocyte infusions (DLI), expansion, or depletion of T-cell subsets (e.g., regulatory T cells), or selection of NK and dendritic cell subsets in order to augment GVT effects while minimizing GVHD) (2).
DONOR ORIGIN OF HEMATOPOIETIC STEM CELLS
• Autologous HCT or “high-dose chemotherapy with autologous stem cell rescue” refers to the collection from and subsequent reinfusion of hematopoietic progenitor cells into a patient with a solid or hematopoietic malignancy. Preparative intensive-conditioning therapy is given in an effort to reduce the number of malignant cells or to achieve immunoablation in a refractory autoimmune disease. Infusion of the patient’s own hematopoietic cells follows in order to rescue the patient from the negative effects of myeloablation, namely unacceptable levels of subsequent infectious and hematologic complications.
• Allogeneic HCT refers to the rescue and replacement of hematopoietic stem cells from an HLA-matched or partially matched donor source different from the patient. Allogeneic HCT may occur after myeloablative, reduced-intensity, or nonmyeloablative conditioning therapy. In addition to the cytoreductive effects of the preparative therapy, allogeneic HCT confers a potentially potent immunologically mediated response of donor immune cells against host tumor cells (i.e., a GVT effect induced by the interaction of donor T cells with host minor or major histocompatibility antigens or tumor antigens).
STEM CELL SOURCES FOR ALLOGENEIC HCT
Preference has historically been given to “matched” related donors (MRD) who are HLA-identical to the recipient, as determined by molecular class I and II HLA typing. As each sibling inherits two haplotypes, one from each parent, there is a 25% chance that any two full siblings will be genotypically identical. Only 30% of patients will have a matched sibling donor. Therefore, alternative, non-HLA-identical related and unrelated donor sources have been increasingly utilized, now enabling the majority of those without sibling donors to undergo HCT. Because of the increasingly varied sources of progenitor cells transplanted, hematopoietic cell transplantation (HCT) is a more representative term than BMT to describe the current field. Stem cell sources include:
• HLA molecularly “matched” unrelated donors (MUD)
• Unrelated donors molecularly “mismatched” at one or more HLA molecules (MMUD)
• Haploidentical-related donors (i.e., children or siblings, sharing only one inherited HLA haplotype)
• Partially HLA-matched umbilical cord blood (UCB)
Hematopoietic progenitor cells capable of restoring hematopoiesis and immune function following transplantation can be procured from:
• Bone marrow (BM; following a bone marrow harvest procedure in the operating room).
• Peripheral blood (PB; following “mobilization” with chemotherapy and/or recombinant hematopoietic growth factor(s) and/or CXCR4 antagonists followed by “collection” by pheresis).
• Umbilical cord (UCB; following full-term delivery) Given longer times to engraftment and higher rates of infectious complications with use of a single cord, the use of two umbilical cord blood units partially matched to each other is now common in adult HCTs.
CD34, a surface glycoprotein expressed on a small percentage of bone marrow cells is typically employed as a surrogate immunophenotypic marker for the pluripotent hematopoietic stem cell. Suitable numbers of CD34-expressing cells are needed in progenitor cell products to ensure acceptable kinetics of engraftment and sustained hematologic recovery following transplantation. Doses of more than or equal to 2 × 106/kg CD34-expressing progenitor cells are typically required in autologous or adult allogeneic donor products. Requirements are approximately one log lower for umbilical cord products.
PRETRANSPLANT CONSIDERATIONS
Designing an HCT approach for a particular patient requires consideration of the underlying disease and the recipient’s medical comorbidities, in addition to details of the transplant itself (3).
• Disease state: The achievement of disease control is a significant if not the dominant determinant of overall survival in most studies and is usually pursued maximally prior to transplantation. For example, according to outcomes analyses from the Center for International Bone Marrow Transplant Registry (CIBMTR), a patient with AML in first complete remission who undergoes an allogeneic transplant from a matched related donor has a 3-year survival probability of 53% compared with a patient with AML not in remission, whose 3-year survival probability is 24% (1).
• Recipient: Morbidity and mortality can also arise from transplant-related complications including organ failure secondary to the conditioning regimen, bacterial or viral infections, and both acute and chronic GVHD (in the allogeneic setting) or their treatments. These can lead to peritransplant mortality rates in the range of 1%–5% after autologous HCT and 10%–40% after allogeneic HCT. In recent years, the number and age of eligible recipients have increased significantly through the use of non-myeloablative and reduced-intensity conditioning regimens with associated lower rates of nonrelapse mortality. In analyses of reduced-intensity conditioning regimens, age per se has not been shown to correlate with worse outcomes, and in 2009 more than 30% of allogeneic stem cell transplantations (SCTs) were performed in recipients over the age of 50, even in patients into their seventies (1). In contrast, performance status and comorbidities, specifically pulmonary, cardiac, and hepatic function, have been shown to have an important impact on nonrelapse mortality based on several validated scoring systems in both myeloablative and nonmyeloablative settings (4). Nevertheless, even after successful transplantation, life expectancy, immune function, and quality of life of recipients remain lower than that of age-matched peers.
PREPARATIVE THERAPY AND DONOR SELECTION
Choices of intensity of the conditioning regimen, donor, and type of stem cell source are driven not just by age and availability but more importantly by: (1) the status and nature of the underlying malignancy, (2) the combination of host comorbidities and treatment toxicities, (3) the reliance on GVT effect needed to prevent relapse in each case, and (4) the desire to avoid complications of GVHD.
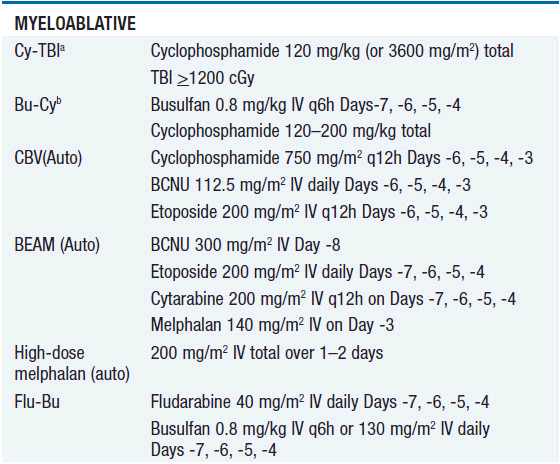
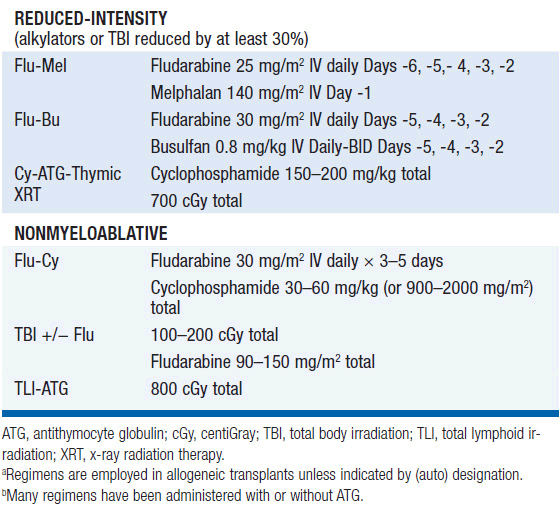
• Intensity of the conditioning regimen: The options for intensity of conditioning regimens have diversified significantly in the last few years, expanding the eligible recipient pool but also increasing potential confusion over classification. One consensus scheme identifies myeloablative (MA) regimens as those that cause irreversible cytopenias requiring hematopoietic cell support, reduced-intensity (RIC) regimens as those that cause cytopenias of variable duration that may or may not be irreversible, and nonmyeloablative (NMA) regimens as those that lead to minimal cytopenias that will recover without hematopoietic cell support (5). Representative regimens for each intensity level are shown in Table 38-2.
RIC or NMA conditioning may be more appropriate for an older patient with a malignancy sensitive to a GVT effect, such as an indolent non-Hodgkin lymphoma. However, with less-potent conditioning, the benefit of decreased nonrelapse mortality may be negated by an increased risk of relapse. Reduced-intensity conditioning may be an inferior approach for younger patients with more aggressive hematologic malignancies such as AML. The presence of a GVT effect, upon which NMA and RIC conditioning regimens rely, has been indirectly demonstrated in specific disease settings in the form of higher rates of relapse observed when progenitor cells are received from an HLA-identical twin sibling, lower rates of relapse seen in the presence of GVHD, induction of remission by abrupt withdrawal of immunosuppression and directly observed when remission is reestablished after infusion of donor leukocytes in the setting of relapse (6). A schematic representing the relative role of GVT against various malignancies and the relative potencies of various conditioning regimens is provided in Figure 38-1. No recommendation for pairing of any disease entity with a specific conditioning regimen is implied, but RIC and NMA regimens are now increasingly employed in older patients whose diseases are more sensitive to GVT. While in some disease settings conditioning regimens of differing intensities have yielded similar overall survival, prospective trials are ongoing to define the disease and age subgroups that will benefit most from a particular conditioning regimen.
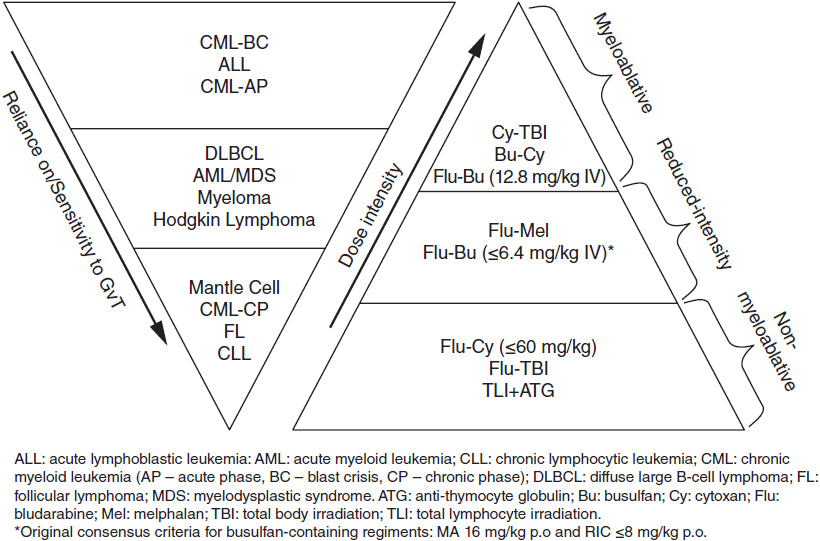
Figure 38-1 Intensities of graft-versus-tumor effect and conditioning regimens.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree