Keywords
autotransplantation, fertility preservation, malignant cell contamination, outcome, ovarian tissue cryopreservation
7.1
Overview
The survival rate among the ~2% of women of reproductive age who have suffered from invasive cancer has substantially increased. Unfortunately, the necessary chemo- and radiotherapy carry the risk of unwanted side effects such as permanent infertility, jeopardizing a woman’s chances of having her own biological children.
Until recently, in vitro fertilization (IVF) and embryo transfer (ET) in combination with cryopreservation were considered the only options for women to conceive after recovery from a sterilizing cancer treatment. However, these methods carry some drawbacks and cannot sustain long-term fertility. They require controlled ovarian stimulation, and are not applicable to prepubertal girls. Cryopreservation and transplantation of ovarian tissue fulfils a number of these shortcomings. Grafting of cryopreserved ovaria.;n tissue can restore menstrual cyclicity, does not require pretreatment and can be performed without delay. Further, the method does not require male gametes and is applicable even to prepubertal girls.
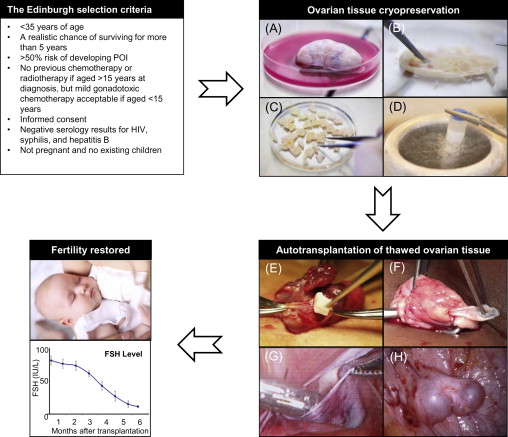
It is very difficult to give a patient an estimation of the risk of premature ovarian insufficiency (POI) due to the number of contributory factors, including age, disease, stage of disease and the fact that the planned chemotherapy treatment often changes during the course of treatment. To help the physicians evaluate each patient, selection criteria such as the Edinburgh criteria ( Figure 7.1 ) can be used for guidance. However, these criteria should merely be used as guidance and not exact guidelines, as each woman should have an individual medical assessment of her ovarian reserve as well as her risk of POI. At the same time, she should have the opportunity to decide whether she is willing to take the risk of the operation in relation to the risk of becoming infertile. In Denmark, there is no exact upper age limit for women having ovarian tissue cryopreserved because some women have a better fertility potential than others (e.g., a high number of antral follicles) despite increased biological age (>35 years of age) and would therefore still benefit from having ovarian tissue cryopreserved. Furthermore, some women are still interested in having ovarian tissue stored even though their own risk of POI is fairly low, as they simply do not want to risk being unable to have children. Other women wish to have their ovary cryopreserved, even when their estimated chances of survival are vague, because the chance of survival is present. When the woman herself needs to cover the cost of cryopreservation, for example as this service is offered in USA, the situation becomes even more difficult. It is therefore of utmost importance to give these women information about their specific situation: their estimated chances of surviving the cancer and furthermore an estimation of their fertility potential, and to inform them that the current experience is rather limited and it is not known to what extent women of, for instance, advanced reproductive age may actually benefit from having tissue transplanted. A parallel situation is seen in IVF treatment, where women of advanced reproductive age receive treatment despite the fact that the success rate is rather low.
7.2
Ovarian Tissue Cryopreservation
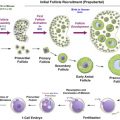
The success of ovarian cryopreservation is based on the high cryopreservation tolerance of small primordial follicles compared to the larger growing follicles. The vast majority of primordial follicles are located in the outermost 1 or 2 mm of the ovarian cortex, which is relatively easy to isolate from the rest of the ovarian tissue (see Figure 7.1 ). The frozen ovarian cortex is stored in liquid nitrogen, allowing time for the patient to recover after cancer treatment is completed.
The most widely used protocol for ovarian cryopreservation is the slow-freezing method, and up until now all live births in humans, except for one birth from Japan, have been achieved after slow-freezing. The most used media compositions include either dimethyl sulfoxide (DMSO) or ethylene glycol, both in combination with sucrose. However, vitrification of ovarian tissue could be one way of improving outcomes after freezing and re-implantation, as new results from non-human primates have shown superior follicular viability and development of antral follicles. Despite these encouraging results, the slow-freezing method is still preferred over vitrification.
Currently there is no consensus as to how much of the ovarian cortex should be harvested for cryopreservation. It is recommended that oophorectomy should be performed on patients receiving high doses of alkylating agents, patients undergoing pelvic irradiation or total body irradiation, and younger prepubertal girls due to the small size of their ovaries. For other patients, 4–5 ovarian cortical biopsies are harvested in some countries, whereas a unilateral oophorectomy is carried out systematically in other countries. The advantages of taking a whole ovary include minimizing the possibility of post-operational complications, e.g., bleeding, and also increasing the possibility of either grafting a large pool of follicles, which potentially should provide fertility with a higher efficacy, or performing repeated transplantation in case the tissue of the first transplantation becomes exhausted. However, the decision of excising one whole ovary in contrast to biopsies remains an issue of debate.
Transportation of ovarian tissue prior to cryopreservation allows hospitals without cryopreservation expertise to treat women locally for the cancer disease and just send the ovarian tissue to the centre that performs cryopreservation. This facilitates quality control, proper equipment, and personnel to fulfil clinical, legal and scientific standards required for proper conduction of the procedure. The feasibility of centralized cryobanking has been proven in Denmark and Germany, where ovarian tissue has been transported up to 20 hours prior to freezing, and these principles are now being introduced in many other countries worldwide. The applicability of this approach will, however, be determined by local regulations.
7.3
Transplantation of Cryopreserved Ovarian Tissue
Ovarian grafts transplanted to women who have entered menopause are able to re-establish a cyclic endocrine hormone milieu including preovulatory follicular development, appropriate conditions for conception, gestation and parturition, sometimes via IVF–ET.
There are two main approaches for transplantation of human ovarian tissue (see Figure 7.1 ). In the orthotopic transplantation, a cortical fragment is grafted either to the remaining postmenopausal ovary, the broad ligament or to the ovarian fossa, whereas heterotopic transplantation refers to a graft site in a sub-peritoneal pocket outside the pelvic cavity, e.g., on the abdominal wall or subcutaneously, such as on a forearm. Most often, tissue is transplanted to the remaining in situ menopausal ovary either after decortication or by placing it under the cortex in order to facilitate a spontaneous pregnancy. Positioning of ovarian cortical pieces is most often performed with the original medulla-site inward and the original cortex-site outward in order to imitate the anatomically correct structure of the ovary. Grafting of the ovarian tissue to the anterior abdominal wall or to the lateral pelvic wall may be necessary if the remaining ovary is removed or has become very small. The general consensus is to transplant ovarian tissue to an orthotopic site, as it provides the optimal environment for follicular and corpus luteum development, compared to heterotopic sites: this means that temperature, pressure, paracrine factors and blood supply are similar to those observed during physiological conditions. Even if transplanting ovarian tissue to heterotopic sites has some advantages, only one live birth has been reported following this procedure.
The size of the graft is also of importance in order to achieve a successful ovarian endocrine function. Meirow and co-workers illustrated two different surgical techniques, one with three ovarian tissue strips (5×15 mm) and a second technique with ovarian cubes in small cavities. Only the ovarian tissue strips regained endocrine function and led to a successful pregnancy with a live birth, whereas the grafted small cubes did not become active. Since that study, a general consensus has emerged to re-implant pieces of 5×5 mm or even bigger tissue strips of 5×15 mm (see Figure 7.1E,F ). According to our experience, larger strips ease the technical challenges of keeping the thawed tissue correctly in place at the graft site and allow firm, stable positioning of each individual piece of cortex, which is likely to facilitate revascularization.
Finally, the quantity of tissue transplanted depends on the desired function of the tissue. Over the years, experience and follow-up studies have developed a consensus to transplant less ovarian tissue for women who wish to regain their menstrual cycle with just one preovulatory follicle per cycle and more ovarian tissue for those who wish to become pregnant.
7.4
Outcome of Transplantations
On a worldwide basis it appears that at the time of writing 39 children have been born subsequent to transplantation ( Table 7.1 ). Thirty of these cases have been described in peer-reviewed papers and 37 cases were mentioned in a review by Macklon and co-workers, with additional children being reported since those publications. Out of these live births, only one twin birth from Australia is a product of a heterotopic graft site. A recent study from the authors’ group described two additional pregnancies following heterotopic transplantation; however, one ended in early abortion and the other after premature preterm rupture of membranes at 19 weeks’ gestation. The latter was probably due to the fact that the patient had received large doses of irradiation to the pelvis in connection with cancer treatment. It is emphasized that pregnancies from heterotopic grafted tissue require intensified luteal phase support and should be considered obstetrical high-risk pregnancies. In addition, two groups have been able to induce puberty by re-implanting frozen-thawed prepubertal ovarian tissue in two young girls. This demonstrates the wide range of possibilities this method offers.
Three different European centres (from Belgium, Denmark and Spain) have collected and evaluated the results of 60 orthotopic re-implantation cases. Fifty-one of the 60 patients had a follow-up >6 months after. The study showed that 93% of the women showed restoration of ovarian activity. Eleven of these 51 patients became pregnant and, at the time of the follow-up, six had already given birth to 12 healthy children. In addition, >50% of the women who became pregnant were able to conceive naturally, which favours orthotopic transplantation. Moreover, the age of patients at the time of cryopreservation has previously been reported to be a predictive factor for pregnancy and the majority of pregnant women were actually under the age of 30 years.
In three of the women who did not become pregnant no follicles were found in the grafted tissue, explaining why ovarian function did not resume. This highlights the importance of evaluating the presence of follicles before re-implantation. Ovarian activity is normally restored within 3.5 to 6.5 months post-grafting, which concurs with the period of follicle growth from the primordial to the antral stage.
In a recent study by Greve and co-workers, 12 women who had thawed ovarian tissue transplanted received assisted reproductive techniques (ART) and had 65 oocytes retrieved as a result of 72 cycles. The outcome per cycle was a pregnancy rate of 6.9% and a live-birth rate of 3.8%. The low pregnancy rate was comparable with women 42 years old even though the oocytes originated from much younger women. It was suggested that the poor outcome reflected reduced follicular selection, rather than aged or damaged oocytes. This explanation parallels the normal decline in female fecundity with increasing age. Additionally, in this study, markers of follicle growth were in the majority of cases only found at very modest levels, as anti-Müllerian hormone remained undetectable in many patients, and levels of inhibin-B were found to be very low. In a study by Dolmans and co-workers, four patients who were between the ages of 21–28 years at the time of cryopreservation had 21 oocytes retrieved. The study found an empty follicle rate per retrieval of 29% (6/21), a tendency to a higher number of abnormal or immature oocytes and a lower embryo transfer rate, when compared to women who had not had their ovarian tissue cryopreserved. Thus, the ovarian reserve is indeed very low following transplantation of thawed ovarian tissue and, at present, it is not possible to know whether the follicles have suffered from cryo injury or whether the low pregnancy rate reflects poor follicular selection.
In IVF treatment, the success rate is measured in the number of oocytes retrieved, the embryo transfer rate, the implantation rate and, of course, the clinical pregnancy rate per transferred cycle; however, these measurements are not always applicable to re-implanted cryopreserved ovarian tissue. Some women have ovarian tissue transplanted because they wish to avoid menopause and not because they have a wish to become fertile; some patients divorce their partner upon transplantation; and some women have a low, insufficient ovarian reserve in the remaining ovary. Furthermore, in contrast to IVF treatment, a pregnancy may occur as long as menstruation still occurs; the authors have, for instance, experienced pregnancies 5 years after grafting.
7.5
Longevity of Grafts
In the study by Greve and co-workers, the longevity of functioning transplants was found to be between 9 months and 7 years. Figure 7.1H shows two human antral follicles at a heterotopic graft site several years after transplantation of thawed ovarian tissue, demonstrating successful grafting and integration of the tissue. Other studies have shown that the transplanted grafts have a mean longevity of approximately 4–5 years when the follicular density was well preserved, but follicular activity can persist for up to 7 years. Given that the longevity of the tissue is good and that in many cases the women have enough tissue stored for 2–3 transplantations, the cryopreserved tissue could be enough to restore endocrine function until the natural age of menopause.
There are several important factors influencing the longevity of the grafted ovarian tissue: First of all, the follicular density of the transplanted tissue at the time of cryopreservation is of great importance. The follicular density depends, besides individual diversity, on the age of the patient at the time of cryopreservation, as the pool of follicles decreases with age. Secondly, chemotherapy before cryopreservation of ovarian tissue has an impact on the ovarian reserve, depending on the gonadotoxicity of the chemotherapeutic agents. Furthermore, the time between transplantation and revascularization of the tissue is critical for the number of surviving follicles. Baird and co-workers found that 60–70% of the follicles were lost in studies on transplantations in sheep. Others have found that >50% of the primordial follicles are lost during and after ovarian transplantation. This is mainly due to ischaemia in the tissue until angiogenesis has been established after transplantation.
Different publications have suggested that using angiogenetic and antiapoptotic factors (such as vascular endothelial growth factor or sphingosine-1-phosphate) prior to grafting could stimulate revascularization of the graft and thereby reduce ischaemia and oxidative stress. Another important factor to facilitate angiogenesis is to fixate the grafted tissue properly to the graft site. This can be done by transplanting the thawed tissue in a subcortical or a subperitoneal pocket. Microsurgical techniques can also be used to suture the graft onto the ovary after decortication followed by fixation of the graft with Interceed® or fibrin glue. By keeping the grafted tissue in place and providing some pressure on the tissue, angiogenesis is believed to be enhanced, as it reduces the breakage of small blood vessels between the graft and graft site when the patient moves around.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree