Abstract
The ovary is a dynamic organ that undergoes some of the most dramatic changes in structure and function of any adult human tissue. The follicles are the major endocrine and reproductive compartments of the ovary, and their health and numbers determine both reproductive potential and reproductive lifespan. The multiple cellular components of follicles interact in a highly integrated manner to secrete sex steroids and protein hormones, and control oocyte development. The follicle-derived hormones act both locally as autocrine and paracrine factors to modulate the actions of gonadotropins and, in some cases, as endocrine signals to program the secretion of gonadotropins that promote follicular development and entrain an ovulatory surge of luteinizing hormone when follicular maturation is completed. They synchronously prepare and sustain the reproductive tract for conception, implantation, and early pregnancy. The oocyte is a large and rare cell that is both totipotent and highly specialized in that it is capable of undergoing meiosis and fertilization with the resulting formation of a new human being. Remarkably, some of these attributes can be suspended for years, only becoming operative when a lengthy process of maturation has been completed. Although many follicles initiate development, only some 400 to 500 follicles or less than 1% of the endowment make the journey to ovulation. Factors that govern the seemingly profligate expenditure of follicles during ovarian development and reproductive life are being defined, mainly through the use of mouse models. Factors identified to date include genes governing formation of primordial germ cells, their migration to the genital ridge, their proliferation, and their incorporation into primordial follicles. Among factors that control entry of primordial follicles into the growing pool are anti-müllerian hormone (AMH) and the Akt and mammalian target of rapamycin (mTOR) signaling pathways. Human mutations associated with primary ovarian insufficiency and studies of aging ovaries and material collected during assisted reproduction procedures have also contributed to the understanding of key genes and mechanisms of age-related ovarian dysfunction (e.g., hypoxia). Biomarkers of ovarian reserve and therapeutic approaches to ovarian insufficiency have emerged from the elucidation of gene functions and the pathways they control.
Keywords
Atresia, anti-müllerian hormone, bone morphogenetic protein 15, corpus luteum, cumulus cell, estradiol, follicle, follicle stimulating hormone, gonadotropin, granulosa cell, growth differentiation factor 9, inhibin, luteinizing hormone, luteolysis, meiosis, menopause, oocyte, ovarian reserve, ovulation, primary ovarian insufficiency, progesterone, theca cell, zona pellucida
Introduction
The ovary is a dynamic organ that undergoes some of the most dramatic changes in structure and function of any adult human tissue ( Fig. 8.1 ). Follicles at all stages of development, from tiny primordial follicles to large preovulatory, Graafian follicles and postovulatory corpora lutea coexist throughout the reproductive lifespan. These structures interact with each other in a paracrine fashion through generation of secretory products including sex steroids and protein hormones. The multiple cellular components within the follicles also interact in a highly integrated manner to control oocyte growth and maturation. This chapter describes the development of female germ cells and follicles, how follicle function is coordinated to support oocyte development and steroidogenesis, and the process of ovarian aging.
Primordial Germ Cells
- ◆
Primordial germ cells are established in extraembryonic tissues at around 3 weeks after fertilization in response to transforming growth factor-β (TGF-β) family signals secreted by surrounding tissues.
- ◆
Transcription factors, pluripotency factors, ligand/receptor signaling pairs, and microRNAs participate in regulating development and migration of primordial germ cells.
- ◆
Primordial germ cells proliferate as they migrate from the yolk sac to the gonadal ridge where they enter the developing ovary.
The primordial germ cell lineage is established early in development, originating in the proximal region of the epiblast, close to the extraembryonic endoderm. A small number of cells emerge under the influence of inductive signals from the extraembryonic ectoderm delivered by members of the TGF-β superfamily, including bone morphogenetic protein (BMP)-2, BMP-4, and BMP-8B (see Chapter 6 ). The WNT signaling pathway ligand, WNT3, must be expressed in the proximal epiblast cells to promote their competence to respond to BMP signals. WNT3 signaling induces β-catenin-mediated transcription of the mesodermal transcription factor T (also known as brachyury), which then activates expression of downstream genes including Prdm1 (previously known as Blimp1 ), Prdm14 , and Tfap2c that are required for primordial germ cell specification.
Primordial germ cell precursors must express SMAD1, SMAD5, and SMAD8, which are phosphoprotein downstream mediators of BMP signaling. Absence of these SMAD proteins results in a marked reduction in the founder cells of the germ cell lineage. The Bmp–Smad gene dosage is critical, and distortion of the ratios impairs germ cell development. Mice homozygous for null mutations of the Bmp2 , Bmp4 , Prdm1 , and Prdm14 genes do not generate primordial germ cells.
Primordial germ cells are identifiable as early as the end of the third week of human gestation by their large size and clear cytoplasm, which contains fewer organelles than the endoderm cells. In mice, the expression of the interferon-induced transmembrane proteins Ifitm1, Ifitm3 (previously known as Fragilis ), and Prdm1 signals their emergence, with the expression of Prdm14 and Dppa3 (previously known as Stella ), marking the founder primordial germ cells. PRDM1 coordinates the repression of genes normally expressed in somatic cells, and both PRDM1 and PRDM14 have roles in maintaining primordial germ cell pluripotency. CBFA2T2 is a scaffolding protein that serves to promote PRDM14-mediated gene repression at specific DNA regions and without which, PRDM14 does not function properly in primordial germ cells.
In addition to these protein regulators, microRNAs, which are short RNA sequences that regulate gene expression mainly by transcriptional and posttranscriptional gene silencing, also control primordial germ cell specification. LIN28, an RNA-binding protein that represses the Let7 microRNA processing pathway, is expressed in founder primordial germ cells and prevents Let7 -mediated suppression of Prdm1 . Other markers of primordial germ cells include tissue-nonspecific alkaline phosphatase and POU5F1 (previously known as OCT4), a transcription factor present in embryonic stem cells and primordial germ cells.
Although most of the above information regarding how primordial germ cells are specified was derived from experiments in mouse models, a global gene expression analysis of individual human primordial germ cells from 4 to 19 weeks post fertilization indicates that mouse and human primordial germ cell transcripts at comparable developmental stages are highly similar. For example, human primordial germ cells express POU5F1 , KIT , T , DPPA3 , LIN28A , PRDM1 , and PRDM14 . One exception is that mouse primordial germ cells utilize SOX2 as an upstream transcription factor, whereas human primordial germ cells utilize SOX17 and possibly SOX15. However, the overall minimal differences suggest that human primordial germ cell specification likely works in a fashion highly similar to that in the mouse.
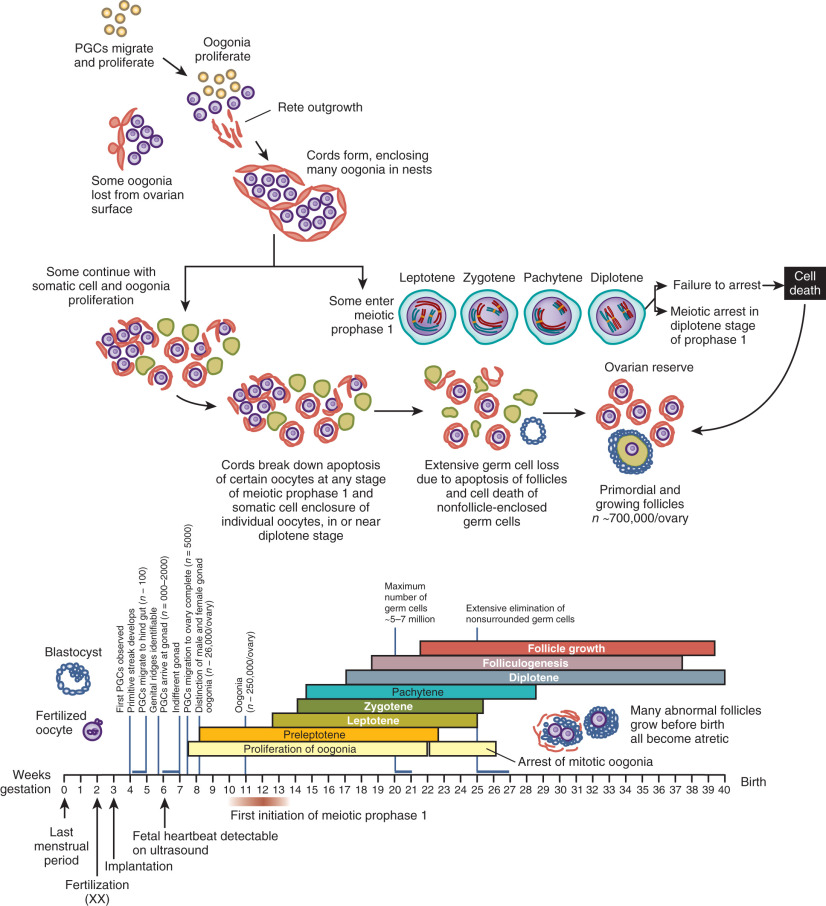
Once specified, primordial germ cells enter a period of migration and proliferation ( Fig. 8.2 ). In the human, they migrate from the yolk sac epithelium to the hindgut and then move through the dorsal mesentery along autonomic nerve fibers and Schwann cells, finally reaching the genital ridge by approximately 4 weeks post fertilization. The cells change during this migration from a “resting” morphology, taking on an irregular shape with protrusions and pseudopodia required for active amoeboid movement.
Primordial germ cell proliferation is characterized by incomplete cytokinesis, resulting in clusters of cells known as “oocyte nests” that form a network because of cytoplasmic continuity via intracellular bridges. Genes involved in primordial germ cell proliferation in mice include Tiar1, which encodes the RNA-binding protein TIAR23, and Fancl (Fanconi anemia, complementation group L), the gene responsible for the mouse “germ cell-deficient” mutation. Fancl encodes a subunit of the Fanconi anemia nuclear protein complex that functions in DNA damage repair. In addition, leukemia inhibitory factor (LIF) and related cytokines appear to be responsible, in part, for primordial germ cell proliferation.
The movement of primordial germ cells to the gonadal ridge is under the direction of a number of other genes. The transcription factor LHX1 maintains proper positioning of primordial germ cells in the hindgut. The two interferon-induced transmembrane proteins expressed in primordial germ cells regulate repulsive (IFITM1) and homing (IFITM3) activities involved in the migration process, although these proteins are not essential for the success of migration. Two ligand/receptor signaling pathways, Kit ligand/Kit receptor tyrosine kinase and Sdf1 (previously Cxcl12)/Cxcr4, are required for primordial germ cell migration. Wnt5a , which encodes a secreted morphogen, also provides a migration cue for primordial germ cells. The gonadal ridge is absent or does not develop normally in mice homozygous for null mutations in the transcription factors Wt1, Nr5a1, Lhx1, Lhx9, and Emx2 ( Table 8.1 ). In addition, nullizygous mice have other abnormalities because some of these genes regulate renal or adrenal development.
| Process | Gene (Aliases) | Protein Type |
|---|---|---|
| Gonadal ridge formation | Wt1 | Transcription factor |
| Nr5a1 ( Sf1 ) | Transcription factor | |
| Lhx1 ( Lim1 ) | Transcription factor | |
| Lhx9 | Transcription factor | |
| Emx2 | Transcription factor | |
| Wnt4 | Secreted ligand | |
| Primordial germ cell specification | Bmp2 | Extracellular growth factor |
| Bmp4 | Extracellular growth factor | |
| Bmp8b | Extracellular growth factor | |
| T (brachyury) | Transcriptional regulator | |
| Tfap2c (AP2 gamma) | Transcription regulator | |
| Smad1 | Transcription regulator | |
| Smad5 | Transcription regulator | |
| Smad8 | Transcription regulator | |
| Primordial germ cell migration | Sdf1 ( Cxcl12 ) | Chemokine |
| Cxcr4 | Chemokine receptor | |
| Ifitm1 (Fragilis) | Transmembrane protein | |
| Ifitm3 (Fragilis 2) | Transmembrane protein | |
| Itgb1 | Cell adhesion | |
| Kitl ( Steel , SCF ) | Transmembrane growth factor | |
| Kit ( W , c-kit ) | Tyrosine kinase receptor | |
| Primordial germ cell development | Prdm1 ( Blimp1 ) | Transcription repressor |
| Prdm14 | Transcription regulator, epigenetic reprogramming | |
| Cbfa2t2 ( Mtgr1 , Zmynd3 ) | Transcription regulator | |
| Dppa3 ( Stella , PGC7 ) | Epigenetic reprogramming | |
| Klf2 | Transcription factor | |
| Nanog | Transcription factor | |
| Sox17 | Transcription regulator | |
| Stra8 | Unknown | |
| Meioc | RNA-binding protein | |
| Atm | Kinase, functions in DNA repair and cell cycle control | |
| Dazl ( Dazla ) | RNA-binding protein | |
| Tial1 ( Tiar ) | RNA-binding protein | |
| Primordial germ cell proliferation and survival | Dnd1 ( Ter ) | RNA-binding protein, microRNA regulator |
| Apobec3 | RNA-binding protein, microRNA regulator | |
| Fancl ( Pog ) | Ubiquitin ligase, DNA damage repair | |
| Pou5f1 (Oct4) | Transcription factor | |
| Zfx | Transcription factor | |
| Primordial follicle formation | Kitl ( Steel , SCF ) | Transmembrane growth factor |
| Kit ( W , c-kit ) | Tyrosine kinase receptor | |
| Figla | Transcription factor | |
| Rac1 | GTPase, signaling protein | |
| Follicle growth | Kitl ( Steel , SCF ) | Transmembrane growth factor |
| Kit ( W , c-kit ) | Tyrosine kinase receptor | |
| Figla | Transcription factor | |
| Foxo3 | Transcription factor | |
| Rb1 | Cell cycle protein | |
| Lkb1 | Kinase | |
| Nobox | Transcription factor | |
| Gdf9 | Growth factor | |
| Bmp15 | Growth factor |
The microRNA cluster, Mir290-295 , is highly expressed in primordial germ cells, and female mice lacking Mir290-295 have premature ovarian failure due to primordial germ cell migration defects. The downstream targets of these microRNAs have not been identified and could include some of the protein regulators mentioned above. The cell adhesion protein integrin β1 must be expressed on the primordial germ cell surface for successful migration to the gonadal ridge. Primordial germ cells that migrate to aberrant locations undergo apoptosis and so are lost from the future ovarian reserve.
Ovarian Morphogenesis
- ◆
Germ cells play an indispensable role in the induction of gonadal development.
- ◆
Two functional X chromosomes are required for normal ovarian morphogenesis.
- ◆
Three competing processes—mitosis, meiosis, and atresia—determine the temporal pattern of germ cell accumulation and decline in the prenatal ovary.
- ◆
Primordial follicles form around 20 weeks of gestation.
Germ cells play an indispensable role in the induction of gonadal development. In the absence of two functional X chromosomes, as in Turner syndrome (45,X0 or other X chromosome structural abnormalities), primordial germ cells can proliferate and migrate to reach the developing gonad, but the germ cells fail to survive because pregranulosa cells are not maintained. As a result, streak gonads containing only stromal cells are present by the time of birth in classical cases, although some germ cells remain in individuals who are mosaics.
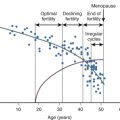
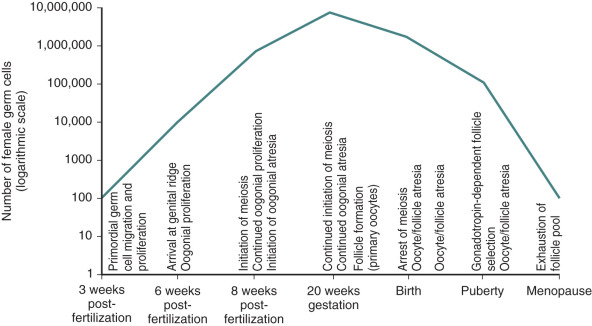
Germ cells that arrive at the gonadal ridge are referred to as oogonia. By 6 to 7 weeks of intrauterine life, the oogonia population has expanded by mitosis to reach some 10,000 cells, and it reaches approximately 600,000 cells by 8 weeks of intrauterine life ( Fig. 8.3 ). However, from this juncture, the oogonial endowment is influenced by three concurrent processes: mitosis, meiosis, and oogonial atresia. As a result of the combined impact of these processes, the number of germ cells peaks at 6 to 7 million by 20 weeks of gestation, and then declines.
Between weeks 8 and 13 of fetal life, some of the oogonia enter prophase of the first meiotic division (see Fig. 8.2 ). Entry into meiosis is triggered by the presence of STRA8, a protein of unknown function. Expression of Stra8 was previously thought to occur in response to retinoic acid signaling; however, recent data indicate that an alternative (nonretinoic acid) activator derived from the mesonephros underlying the fetal gonad is responsible. Meiotic entry requires the presence of deleted in azoospermia-like (DAZL) as a “licensing” factor. In addition, a newly identified protein named MEIOC stabilizes mRNA transcripts that encode proteins that function during meiosis. If correctly executed, meiosis appears to provide temporary protection from oogonial atresia. Oogonia that persist beyond the seventh month of gestation without entering meiosis undergo programmed cell death.
Primordial follicle formation begins at around 20 weeks of gestation and is accompanied by oocyte nest breakdown as the intercellular bridges are lost and pregranulosa cells surround the primary oocytes (see Fig. 8.2 ). Many of the genes that regulate primordial follicle assembly are shown in Fig. 8.4 . This process is regulated by signaling between the germ cells and somatic cells via the Notch-Jagged and KIT-Kit ligand pathways. In addition, TAF4B, a transcription coactivator that is enriched in the fetal gonad, appears to regulate the timing of oocyte nest breakdown and to be essential for oocyte survival. At midgestation, when the ovarian germ cell endowment is at its apex, two thirds of the total germ cells are intrameiotic primary oocytes; the remaining one third are oogonia. One reason for the subsequent decline in germ cell numbers is the declining rate of oogonial mitosis, a process that ends by approximately 7 months of intrauterine life. The reduction in germ cells is also the result of an increasing rate of oogonial atresia, which peaks at about month 5 of gestation, followed by follicular atresia, which begins around the sixth month of gestation. One purpose for the oogonial atresia may be to provide organelles such as mitochondria and other cytoplasmic components to the surviving subset of oocytes within the same germ cell cyst via direct transfer. Apoptosis is believed to be triggered by either a deficiency in survival factors, such as Kit ligand, LIF, or basic fibroblast growth factor (FGF), or by death-inducing factors, such as Fas ligand, TGF-β, and activin.

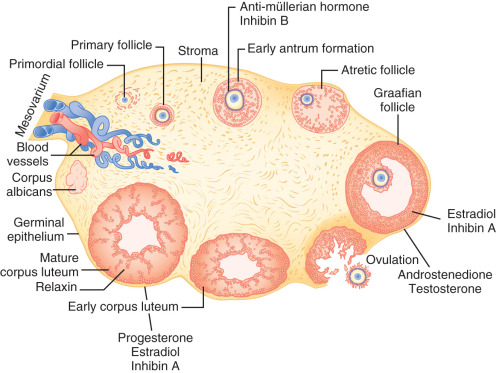
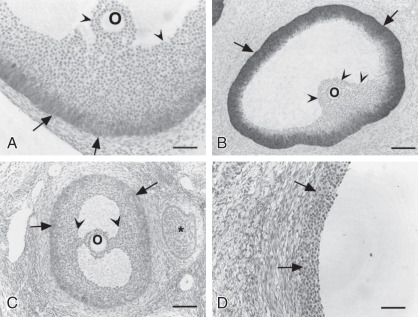
Primordial follicles in the human ovary (30 to 60 µm in diameter) are comprised of a late diplotene primary oocyte (9 to 25 µm in diameter) surrounded by a single layer of flattened granulosa cells ( Fig. 8.5 ). Follicles at this stage of development are not believed to be influenced by gonadotropins. Primary follicles (>60 µm in diameter) are characterized by a primary oocyte surrounded by a single layer of cuboidal granulosa cells. Secondary follicles (<120 µm) consist of a primary oocyte surrounded by several layers of cuboidal granulosa cells (<600 cells), as shown in Fig. 8.5 .
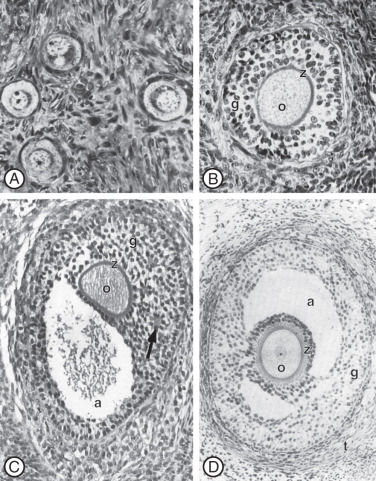
Inactivating mutations in the oocyte-specific transcription factor, folliculogenesis specific basic helix-loop-helix (Figla) , prevent the formation of primordial follicles. Mice homozygous for a null mutation in the X-linked zinc finger gene, Zfx, have a reduced number of oocytes, resulting in a diminished reproductive lifespan. Mutations in the Atm gene, the murine homologue of the human ataxia-telangiectasia gene, disrupt gametogenesis at the leptotene stage of meiosis, and consequently result in sterility. Disruption of Wnt4 alters the expression of steroidogenic enzymes in the ovary, resulting in increased testosterone synthesis and wolffian duct masculinization, and affects germ cell entry into meiosis. WNT4 mutations are associated with müllerian defects (Mayer-Rokitansky-Kuster-Hauser syndrome) and hyperandrogenism in women. A more extensive list of mouse genes involved in ovary development and follicle formation is provided in Table 8.1 .
Relentless and irreversible attrition from midgestation onward progressively diminishes the gonadal germ cell complement, leaving approximately 700,000 primordial follicles in the ovaries at birth. This number decreases further to approximately 300,000 by the onset of puberty; of these follicles, only 400 to 500 ovulate in the course of a reproductive lifespan (see Fig. 8.3 ).
Although there is good evidence that embryonic stem cells can give rise to oocyte-like cells and follicle-like structures in culture, the notion that germ cells and follicles can arise in vivo in the mammalian ovary during postnatal life from an oogonial stem cell population and contribute to the ovarian reserve remains controversial.
Compared with the fetal testis, the human fetal ovary is generally believed to be steroidogenically quiescent, although cholesterol side-chain cleavage activity and 17α-hydroxylase/17,20-desmolase activities are detectable. Even though follicles are present in the fetal and infant ovary, their steroidogenic capacity only becomes evident at puberty.
Epigenetic Programming and Germ Cell Development
- ◆
Primordial germ cells undergo extensive reprogramming of their chromatin structure (epigenetic reprogramming) beginning soon after specification and continuing after entry into the developing ovary.
- ◆
The epigenetic changes include global DNA demethylation and erasure of histone modifications, which removes the inherited somatic cell marks to generate a “naïve” epigenetic state.
- ◆
Complete erasure of genomic imprints occurs during the reprogramming process in preparation for reestablishing female-specific imprints later during oocyte development.
Dramatic alterations in epigenetic programming of primordial germ cells are initiated soon after their specification, and continue after entry into the developing gonad. Nearly complete DNA demethylation occurs across the entire genome, including the inactive X-chromosome, which allows transcriptional activity to resume. Measurements of DNA methylation in human primordial germ cells reveal almost complete demethylation by 10 to 11 weeks of gestation. This global demethylation process is in part passive, in other words, simply a consequence of DNA replication in the absence of DNA methyltransferase activity; thus maintenance methylation cannot be accomplished. An enzyme-mediated active demethylation process also occurs in combination with activation of the DNA base excision repair pathway, which is required to resolve the proper DNA sequences. Posttranslational modifications to DNA-associated histone proteins are either transiently or permanently lost due to a combination of histone replacement by nonmodified histone proteins and repression of histone modifying enzymes such as histone methyltransferases. These modifications are temporally associated with pronounced alterations in nuclear architecture, including chromatin decondensation and an increase in nuclear size. Together, these epigenetic changes serve to almost fully erase the inherited somatic differentiation program, resetting the cells to a “naive” epigenetic state.
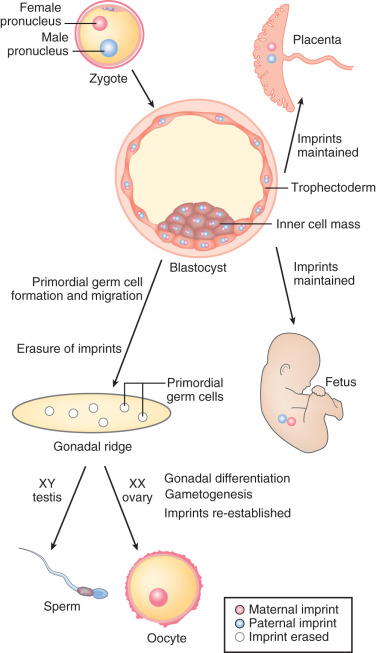
A critical step in mammalian germ cell development, the modification of genomic imprinting, occurs with these epigenetic reprogramming events. In mammals, there is an absolute requirement for the inheritance of a chromosomal complement from both the mother and the father. The basis for this requirement is that specific genes must be expressed from one allele, not both, for successful development. This task is accomplished by a tightly orchestrated set of modifications of the maternal and paternal chromatin that include methylation of CpG sequences of specific “imprinted” genes, depending on the parent of origin; the result is differential transcriptional regulation. Complete erasure of these imprints occurs during the primordial germ cell epigenetic reprogramming process and is required in preparation for switching the inherited maternal and paternal imprinted patterns to reflect the sex of the progeny. In the case of oogonia, both maternal and paternal imprints are erased, and maternal imprints are later established during oogenesis in the fetal ovary and during oocyte growth ( Fig. 8.6 ). Demethylation of imprinted loci appears to occur much later than global genome demethylation, with completion at ~16 to 17 weeks gestation.
The Follicle and Its Surroundings
- ◆
The expression of oocyte-specific genes is essential for normal follicular development.
- ◆
The oocyte and the somatic cells of the follicle engage in a complex conversation involving transfer of metabolites through gap junctions, and an autocrine and paracrine dialogue, mainly through members of the TGF-β family.
- ◆
The ovarian stroma serves as an insulator for growth factors and modulators produced by growing follicles.
- ◆
Ovarian function is influenced by a neural input and the action of neurotrophic factors and tachykinins.
Oocyte
Oocyte Ultrastructure
The oocyte is an extremely large cell that has a large, highly transcriptionally active nucleus also known as the “germinal vesicle.” It is rich in cytoplasm with numerous organelles and proteins. The key task of the oocyte as it grows is to generate all the components required for successful ovulation, fertilization, and the early stages of preimplantation embryo development before transcription begins from the newly formed embryonic genome. Essential cytoplasmic components include ribosomes, mitochondria, and maternal mRNAs that are stored for later translation into proteins required for maturation and embryo development. Ribosomes and maternal mRNAs are found in the cytoplasm associated with fibrillar cytoskeletal structures known as cytoplasmic lattices, which are composed of keratins, tubulin, and other highly abundant oocyte proteins.
Oocyte mitochondria are unique in their structure and DNA content relative to somatic cells in that they are spherical, have few cristae, and contain only one to two copies of mitochondrial DNA each. Oocytes also contain far more mitochondria than somatic cells. Primordial germ cells begin with about 10 mitochondria, and the numbers of mitochondria increase rapidly throughout germ cell migration to the gonad, entry into meiosis, and primordial follicle formation when the primordial oocytes each contain ~6000 mitochondria. With the onset of oocyte growth, mitochondrial replication continues, with an estimated 300,000 to 400,000 mitochondria in fully grown human oocytes. At this stage, the mitochondria form aggregates that are tightly associated with smooth endoplasmic reticulum membranes. The large storehouse of oocyte mitochondria is essential to generate sufficient adenosine triphosphate (ATP) to support oocyte development, maturation, fertilization, and early embryo development.
Oocyte-Specific Genes
The growing oocyte expresses a number of genes that are essential for successful follicular development, fertilization, and preimplantation development. Mouse knockout studies have identified several oocyte-specific transcription factors essential for folliculogenesis. Figla, Sohlh1 (spermatogenesis- and oogenesis-specific basic helix-loop-helix 1), and Lxh8 (LIM homeobox 8) appear to be required for the formation of primordial follicles from naked primordial oocytes, whereas Nobox (newborn ovary homeobox) is involved in the transition of primordial to primary follicles (see Fig. 8.4 ). Dazl , Cpeb1 (cytoplasmic polyadenylation element binding protein 1), and Ybx2 (previously known as Msy2 ) are DNA- or RNA-binding proteins involved in regulating mRNA translation within the oocyte.
Oocyte growth is accompanied by formation of the zona pellucida (ZP), an extracellular matrix surrounding the oocyte. The ZP protects the developing germ cell in the follicle, the ovulated egg in the oviduct, and the cleavage-stage embryo. It also serves as the initial site of contact with sperm, and after fertilization it becomes a barrier that discourages polyspermy. Three genes have been characterized that encode ZP1 (ZPA), ZP2 (ZPB), and ZP3 (ZPC), which are the main sulfated glycoproteins of the ZP. A polymer of ZP2 and ZP3 proteins forms filaments that are interconnected by ZP1, a minor component of the ZP. FIGLA regulates the coordinated expression of these genes during the oocyte growth phase. Humans and rats have a fourth ZP-1 like subunit (ZP4) that is not expressed in mice.
In mice, ZP3 was believed for many years to be the primary sperm receptor, with ZP2 being a secondary receptor. However, there is now compelling evidence that both mouse and human sperm recognize a specific region of the amino terminus of ZP2 that is essential for successful ZP penetration. After fertilization, a cortical granule metalloprotease named ovastacin is released from the egg and cleaves ZP2, which prevents additional sperm from binding the ZP and results in the ZP block to polyspermy.
Mice lacking ZP1 form structurally abnormal zonae and have reduced fecundity. In mice lacking ZP2, a thin ZP forms that is not retained in preovulatory follicles. The antral-stage follicle number is substantially reduced, few eggs are ovulated, and no two-cell-stage embryos can be found in mated animals. Moreover, blastocysts derived from in vitro fertilized oocytes from females lacking ZP2 do not undergo normal development. Mice lacking ZP3 form no ZP despite the expression of the other zona proteins. Few eggs are ovulated, and the females are sterile. As in the ZP2 mutant, in vitro fertilized eggs from ZP3-deficient mice do not develop beyond the blastocyst stage.
Several oocyte-specific “maternal effect” genes have been identified in mouse models that are expressed in the oocyte, but only appear to be required during preimplantation embryo development. Four of these genes encode proteins that form a “subcortical maternal complex” in oocytes, including Nlrp5 (NACHT; leucine-rich repeat; and PYD-containing 5, also known as MATER ), Khdc3 (KH domain containing 3, also known as Filia ), Ooep (oocyte-expressed protein, also known as Floped ), and Tle6 (transducin-like enhancer of split 6). Peptidylarginine disulfide isomerase, type VI (PADI6) may also function in this complex based on its similar localization patterns in the oocyte and cleavage stage embryo. Mouse oocytes lacking NLRP5, PADI6, or OOEP do not have cytoplasmic lattices, are defective in their ability to synthesize proteins, and fail to develop following fertilization. KHDC3 is required in oocytes and early embryos to regulate spindle function; embryos of Khdc3 -null female mice develop poorly because of a high incidence of aneuploidy. Nlrp2 , a gene related to Nlrp5 , is expressed in both oocytes and granulosa cells during folliculogenesis; the maternal protein is required in early embryos for successful development.
Paralogues of the mouse subcortical maternal complex genes are expressed in human oocytes and likely function similarly. Indeed, women homozygous for a point mutation at a phosphorylation site in TLE6 are sterile due to a failure of embryo cleavage following fertilization. Similarly, human oocytes lacking PADI6 undergo developmental arrest following fertilization.
Zar1 (zygote arrest 1) encodes a cytoplasmic protein required for the transition from fertilized egg to cleaving embryo by an unknown mechanism. Gclm (glutamate-cysteine ligase, modifier subunit) encodes a protein that regulates synthesis of glutathione, which is critical for controlling cellular redox status. Mouse embryos deficient in maternal GCLM have deficits in their ability to develop to the blastocyst stage. Npm2 (nucleoplasmin 2) encodes a nuclear protein generated before oocyte maturation that affects heterochromatin organization and histone deacetylation. In the absence of NPM2 , ovulation and fertilization occur normally, but there is a complete failure of preimplantation embryo development. Dppa3, in addition to its role in primordial germ cell specification, is a maternal effect gene required for normal preimplantation embryo development.
Oocyte-Derived Factors and the Control of Follicular Growth and Maturation
The concept that the oocyte plays an important role in follicular function and is far more than a passive inhabitant of the follicle emerged from two observations: (1) that follicular survival depends on the presence of viable germ cells and (2) that removal of the oocyte from an antral follicle is followed by luteinization, indicating that the oocyte produces factors that restrain the terminal differentiation of granulosa cells. Additional support for this concept was derived from experiments in which midsized oocytes from mouse secondary follicles were transferred by a grafting procedure into primordial follicles. This transfer resulted in a doubling of the rate of development of the primordial follicular cells in the presence of the secondary follicle oocyte.
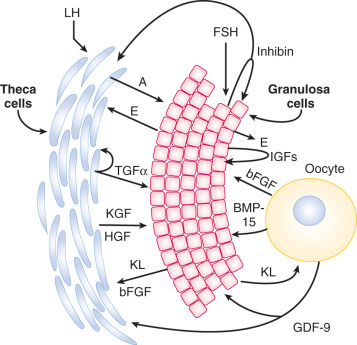
The effects of the oocyte on follicular growth are mediated in part by closely related members of the TGF-β superfamily that are selectively or specifically produced by the oocyte. These factors, growth differentiation factor (GDF)-9 and BMP-15, influence granulosa and theca cell function ( Fig. 8.7 ) (see Chapter 6 ). The importance of GDF-9 and BMP-15 has been established through genetic manipulation of mice and the discovery of spontaneous mutations in the genes encoding GDF-9 and BMP-15, which affect follicular dynamics in sheep. In humans, there is a correlation between higher GDF-9 and BMP-15 expression and the overall quality of the oocyte and subsequent embryo.
Growth Differentiation Factor-9
GDF-9, encoded by a gene on chromosome 5q31.1, is highly expressed by oocytes and, to a lesser extent, by primate granulosa cells. The follicles of GDF-9-deficient mice arrest in growth at the primary stage, yet the oocytes continue to grow at a faster rate than wild-type oocytes, progressing to advanced stages of differentiation seen in the antral follicles of normal mice. However, there are ultrastructural abnormalities in the interconnections between granulosa cells and oocytes; the oocytes ultimately die, leaving a ribbon of zona pellucida behind. The theca also does not form around the follicles, implicating GDF-9 in the organization or proliferation of this follicular component. Studies in the rat also indicate that GDF-9 stimulates the growth of primary follicles, consistent with the block to progression at the primary stage in GDF-9-deficient mice.
GDF-9 has a variety of actions on granulosa cells and theca cells that are species-specific, acting at least in part through interaction with the activin-like receptor (ALK)-5 (TGF-βRI) and BMP receptor type 2 (BMPR-2) receptor complex. In rodents, GDF-9 stimulates granulosa cell differentiation, including induction of luteinizing hormone (LH) receptors and steroidogenesis. In cumulus cells, GDF-9 promotes expression of hyaluronan synthase 2, pentraxin 3, and tumor necrosis factor inducible gene 6 (TSG-6), the latter being proteins that are incorporated into the proteoglycan extracellular matrix of the cumulus oophorus complex. It also suppresses urokinase expression while stimulating COX-2 and prostaglandin synthesis and progesterone formation. LH receptor expression is suppressed by GDF-9, which would discourage luteinization of the cumulus cells. These actions of GDF-9 promote unique phenotypes in the granulosa cells surrounding the oocyte, which are exposed to the highest GDF-9 concentrations. GDF-9 inhibits human theca cell steroidogenesis in vitro. It also stimulates theca cell proliferation, a finding consonant with the apparent role of GDF-9 in the murine ovary in controlling thecal development.
Bone Morphogenetic Protein-15
BMP-15, also known as GDF-9b, encoded by a gene on the X chromosome, is another member of the TGF-β superfamily produced by oocytes. It is related structurally to GDF-9 and shares a similar pattern of expression. Targeted deletion of the murine Bmp15 gene causes a modest ovarian phenotype in nullizygous animals of subfertility with diminished ovulation and fertilization rates. However, mice nullizygous for Bmp15 and heterozygous for a Gdf9 mutation have severely impaired fertility, with abnormalities in folliculogenesis and cumulus cell function. Spontaneous point mutations in the ovine Bmp15 gene (e.g., Inverdale and Hanna sheep) result in phenotypes that differ from those in Bmp15 knockout mice. In the heterozygous state, the number of follicles ovulating is increased, and thus there is an increase in fecundity. However, primary ovarian failure, with a phenotype resembling the murine Gdf9 knockout, is observed in ewes homozygous for the mutations. In vitro, BMP-15 stimulates granulosa cell mitosis. Thus its absence in vivo would be predicted to impair follicular growth, which is consistent with the ovarian abnormalities in homozygous mutant sheep.
BMP-15 binds to a receptor complex consisting of BMPR1B (ALK6) and BMPRII. A point mutation in BMPR-1B in Booroola sheep is associated with an additive increase in ovulation rate, based on the copy number of mutant alleles. Targeted deletion of the Bmpr2 gene in mice does not affect follicular development, but it does yield an infertility phenotype as a result of defects in cumulus cell expansion that prevent in vivo fertilization.
Both BMP-15 and kit ligand participate in a negative feedback loop: BMP-15 stimulates kit ligand expression by granulosa cells, whereas kit ligand inhibits BMP-15 expression in oocytes. In the presence of an oocyte, both BMP-15 and kit ligand stimulate granulosa cell mitosis. The observations that only the oocyte expresses kit, the kit ligand receptor, and that kit ligand suppresses expression of BMP-15, a granulosa cell mitogen, suggest that the oocyte must be involved in producing another granulosa cell mitogen.
GDF-9 and BMP-15 are both synthesized as proproteins that form dimers and are then proteolytically processed to yield the bioactive molecules. Notably, the Inverdale mutation that inactivates BMP-15 dramatically impairs the proteolytic processing of both the mutant BMP-15 and wild-type GDF-9 in coexpressing cells. This observation suggests that the phenotype of the Inverdale sheep may be the result, at least in part, of GDF-9 deficiency due to interference by mutant BMP-15 with wild-type GDF-9 processing. Similarly, cells coexpressing mutant human forms of BMP-15 and GDF-9 associated with premature ovarian failure have decreased production of the mature proteins, likely because of impaired posttranslational processing. Although both GDF-9 and BMP-15 homodimers have biological activity, heterodimers of the two proteins are far more bioactive as indicated by their potency in regulating granulosa cell survival, granulosa cell functions that support oocyte metabolism, and cumulus cell expansion during maturation. These findings suggest that GDF-9:BMP-15 heterodimers, rather than homodimers of the individual proteins, are the essential functional ligands secreted by the oocyte.
Granulosa Cells
Based on studies in murine models, it has been proposed that there are two waves of granulosa cell formation, both originating from the ovarian surface epithelium. The first wave participates in the formation of medullary follicles, the second wave, follicles in the ovarian cortex. The emerging granulosa cells are derived from GATA binding protein 4 (GATA4)-expressing cells. GATA4 works in concert with WNT4, R-spondin 1 (RSPO1), β-catenin (Ctnnb1), and Forkhead Box L2 (FOXL2) to establish the fetal granulosa cells and regulate folliculogenesis.
The cohort of granulosa cells that surrounds each oocyte has an oligoclonal origin, with three to five parent cells giving rise to the full complement of granulosa cells in a mature follicle. Granulosa cells receive no direct blood supply, and because a basal lamina separates them from the vascularized theca interna, there is a relative blood–follicle barrier that restricts the entry of leukocytes and high-molecular-weight substances (such as low-density lipoproteins). The absence of a blood supply also necessitates intimate intercellular contact between neighboring granulosa cells and the oocyte.
Intercellular Communication Between Granulosa Cells and the Oocyte
Granulosa cells are interconnected by an extensive network of gap junctions, which are important for metabolic exchange and transport of small molecules between neighboring cells. The number of gap junctions per granulosa cell increases as follicles develop, coupling them into a functional syncytium. Moreover, granulosa cells extend cytoplasmic processes through the zona pellucida to form gap junctions with the plasma membrane of the oocyte.
Gap junctions are comprised of hexameric arrays of proteins called connexins. Connexin-37 and connexin-43 are two of the most important follicular connexins, and they form gap junctions with different permeability properties. Connexin-37 is the predominant connexin in the oocyte, whereas connexin-43 predominates in granulosa cells. However, at least the first layer of granulosa cells surrounding the oocyte also expresses connexin-37. Communication between granulosa cells and the oocyte occurs through homotypic connexin-37 gap junctions, whereas gap junctional communication between granulosa cells is via homotypic connexin-43 complexes. Follicle-stimulating hormone (FSH), TGF-β1, and all-trans retinoic acid that is synthesized locally by cumulus cells induces connexin-43 expression in granulosa cells. In antral follicles, oocytes are restrained from resuming meiosis via diffusion of cyclic GMP (cGMP) through gap junctions. The ovulatory surge of LH suppresses connexin-43 mRNA expression, and causes mitogen-activated kinase-mediated phosphorylation of connexin-43 protein that ultimately results in gap junction closure and disruption of cell-to-cell metabolic coupling.
The importance of connexins to follicular function was shown in the ovarian phenotype of connexin-37 and connexin-43 knockout mice. In connexin-37-deficient mice, created by targeting the Gja4 gene, follicle growth is arrested at the preantral stage; oocyte growth, although it commences, is also subsequently arrested before meiotic competence is achieved, resulting in loss of oocytes and formation of luteinized structures. Connexin-43-deficient mice, created by targeting the Gja1 gene, have an ovarian phenotype characterized by diminished germ cell number and impaired growth of follicles beyond the primary stage. Other connexins are expressed in the ovary, but their specific functions are unknown.
Granulosa Cells and the Convergence of Multiple Signaling Pathways
Granulosa cells express receptors for, and respond to, a large number of factors that are either generated locally within the follicle or that enter the follicular compartment from the blood. These include oocyte-derived factors, autocrine/paracrine factors produced by the granulosa cells themselves, products of theca cells, and circulating factors derived from the pituitary and other tissues such as fat. The repertoire of signaling molecules that have been shown to affect primate as well as other animal granulosa cells in vitro and in vivo beyond FSH and LH is extensive, encompassing hypothalamic factors (e.g., gonadotropin releasing hormone [GnRH] and kisspeptin), other pituitary hormones (e.g., growth hormone and prolactin), a large array of growth factors (e.g., epidermal growth factor [EGF] family members, TGFβ family members, and insulin-like growth factors [IGFs]), hormones controlling metabolism (e.g., insulin), vascular tone and angiogenesis (e.g., endothelins and apelin), cytokines (e.g., tumor necrosis factor-α [TNF-α]), and adipokines (e.g., leptin, adiponectin). Some of these factors have a critical role as documented from the phenotypes associated with mutations in humans or spontaneous or induced gene mutations in animals such as previously described for GDF-9 and BMP-15. The hierarchical and temporal dominance of the actions of the plethora of factors that can act on granulosa cells has yet to be established.
In addition to the multiple factors that act on the array of receptors expressed on granulosa cells, microvesicles and exosomes participate in communication within the follicle. MicroRNAs are one of the better characterized cargoes of these shed components.
Granulosa Cell Endocrine Activity
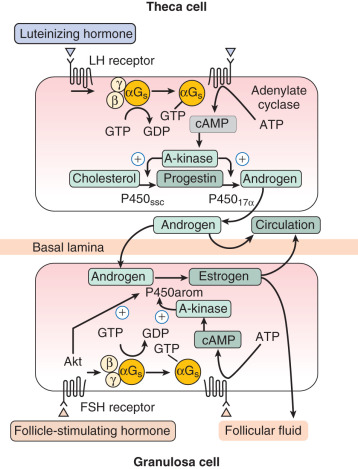
The key steroid hormone produced by the preovulatory granulosa cells is estradiol. The synthesis of estrogens requires a collaborative relationship with adjacent theca cells, which produce the immediate precursors (androgens) for the aromatization reaction (see Chapter 4 ). The control of this process is under the direction of LH, acting on thecal elements, and FSH, acting on the granulosa compartment through multiple signaling pathways including protein kinase A and Akt ( Fig. 8.8 ; see Chapter 2 ). The actions of LH and FSH are modulated by local factors produced by the somatic cells of the follicle and the oocyte as noted above. The two cell–two gonadotropin model is a formidable example of the integrated function of the different cellular components of the follicle.
Studies of isolated granulosa cells have shown that FSH, but not LH, stimulates estrogen production when the cells are provided with an aromatizable substrate. In contrast, isolated human theca cells do not produce substantial amounts of estrogens, but instead secrete dehydroepiandrosterone, androstenedione, and smaller amounts of testosterone when adenylate cyclase activity is stimulated. The aromatase activity of granulosa cells is estimated to be at least 700 times greater in the granulosa cells of large preovulatory follicles than in theca cells, arguing strongly for the cellular compartmentalization of estrogen synthesis outlined in the two-cell–two-gonadotropin model.
Inhibin, a member of the TGF-β protein superfamily, is a heterodimeric 32-kDa glycoprotein composed of two subunits, α and β, linked by disulfide bonds. There is a single common α subunit, but there are different β subunits, denoted β A and β B (see Chapter 6 ). The αβ A and αβ B heterodimers are named inhibin A and B, respectively. In the ovary, the primary source of inhibin is granulosa cells. The main endocrine role for inhibin, for which it was discovered and named, is to suppress pituitary FSH production. In vitro, inhibin augments LH- and IGF-stimulated androgen production by theca cells, and elevated inhibin production by granulosa cells in polycystic ovary syndrome (PCOS) may contribute to the hyperandrogenemia that is associated with this condition.
Although both isoforms of inhibin have similar biological properties, their synthesis is regulated differently during the follicular and luteal phases. Inhibin B is secreted mainly during the early follicular phase, with levels decreasing in the midfollicular phase, and subsequently declining further after the LH surge. Concentrations of inhibin A are low during the first half of the follicular phase, but increase during the midfollicular phase and peak during the luteal phase. The differential production of inhibin A and inhibin B is supported by measurements made on follicles of different sizes that showed that inhibin A levels rise with increasing follicular size while inhibin B levels reach a peak in follicles of 9 to 10 mm.
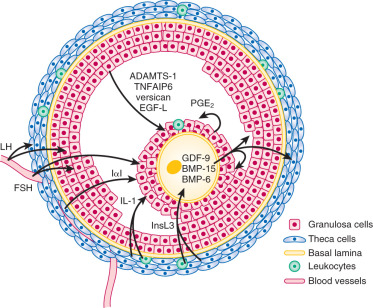
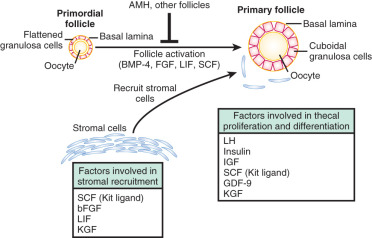
Granulosa cells secrete other protein molecules into the circulation, including anti-müllerian hormone (AMH), a member of the TGF-β family, which plays important roles in follicular dynamics by restraining the entry of primordial follicles into the growing pool (see Chapter 6 ). AMH is expressed by granulosa cells at the time primordial follicles are recruited to grow into the preantral stages. Highest expression is observed in preantral and small antral follicles. Consequently, AMH levels in serum reflect the number of growing follicles in the size range of 2 to 9 mm. In the mouse ovary, the absence of AMH causes primordial follicles to be recruited more rapidly, resulting in the early exhaustion of the follicular endowment. AMH also reduces the sensitivity of follicles to FSH and the number of LH receptors on granulosa cells. Based on its activities (see Chapter 21 ), AMH has been proposed to be involved in the pathophysiology of PCOS. Although some extraovarian tissues express AMH (type II) receptors like the human endometrium, the roles of AMH in female reproduction outside of the ovary have yet to be defined.
Granulosa Cell Phenotypic Heterogeneity
Granulosa cells display different phenotypes within the follicle, depending on their location. The mural granulosa cells located near the basal lamina, granulosa cells lining the antrum, and cumulus granulosa cells each have distinguishing features that are determined, in part, by their proximity to the oocyte and theca cells, and consequently the paracrine substances released by the oocyte and theca cells. The mural granulosa cells in the antral follicle express the greatest steroidogenic activity ( Fig. 8.9 ). In addition, mural granulosa cells in the preovulatory follicle have the highest level of LH receptors. The granulosa cells closest to the antral cavity have a lower expression of steroidogenic enzymes, whereas those in the middle region have greater mitotic activity than the antral and mural granulosa cells.
The cumulus cells, which are released with the oocyte at ovulation, do not express aromatase, and their LH receptor content and level of LH responsiveness are substantially lower than those of their mural counterparts. In mice, they have distinguishing gene expression patterns that include expression of Slc38a3 , which encodes a sodium coupled neutral amino acid transporter, and higher expression of the anti-müllerian hormone gene, Amh . In response to oocyte-secreted GDF-9 and BMP-15, cumulus cells and granulosa cells close to the antrum do not express DDIT4L, a suppressor of mTOR signaling, whereas mural granulosa cells express high levels of this protein. As a result, the cellular metabolism regulator, mTOR, is activated in the cells closest to the oocyte, which improves transfer of nutrients to the oocyte and increases oocyte developmental competence. Cumulus cells proliferate after the LH surge and are active in producing an extracellular matrix consisting of hyaluronan, proteoglycans, and proteoglycan-binding proteins when stimulated by the prostaglandins generated in response to the ovulatory stimulus. The elaboration of this matrix leads to the preovulatory expansion of the cumulus–oocyte complex, which appears to be essential for ovulation ( Fig. 8.10 ). Differential patterns of expression of prostaglandin E (EP) receptors allow granulosa cell subpopulations to respond uniquely to PGE 2 during ovulation (see Chapter 4 ).
Luteinized granulosa cells of the ovulated follicle undergo terminal differentiation to give rise to the large luteal cell population of the corpus luteum. These cells have an increased capacity to synthesize progesterone, resulting from upregulation of the machinery required to acquire cholesterol from the circulating lipoproteins, which now have access to the granulosa-lutein cells as a result of neovascularization of the developing corpus luteum. The granulosa-lutein cells also retain the capacity to synthesize estrogens from androgen precursors produced by theca lutein cells.
Theca Cells
The theca and interstitial cells are believed to arise from fibroblast-like mesenchymal cells in the stromal compartment. They first appear around follicles that have two or more layers of granulosa cells ( Fig. 8.11 ).
Production of GDF-9 by the oocyte is required for development of the theca layer. In the absence of GDF-9, theca cells are recruited to surround the follicle, but do not undergo proper functional differentiation. The Kit and Kit ligand system is thought to play a prominent role in early development of the androgen producing cells of the ovary and testis. Theca cells also express Kit, the receptor for Kit ligand, and researchers have postulated that Kit ligand produced by granulosa cells is important in organizing the theca layer around the developing follicle. Recent studies in the mouse indicate that hedgehog signaling also plays a critical role in establishment of the theca cell lineage. In response to oocyte GDF-9, granulosa cells secrete both desert hedgehog (DHH) and indian hedgehog (IHH); these ligands promote recruitment of theca cells to the follicle and support their differentiation.
Theca cells engage in a bidirectional dialogue with granulosa cells through the production of keratinocyte-derived growth factor (KGF) and hepatocyte growth factor (HGF). KGF and HGF, like FSH, stimulate granulosa cells to produce Kit ligand, whereas Kit ligand acts on the theca cells to promote expression of KGF and HGF in a positive feedback loop (see Fig. 8.7 ). Kit ligand, KGF, and HGF achieve their highest concentrations in large antral follicles. As the oocyte expresses Kit receptor, this feed-forward loop also affects oocyte function. Moreover, theca-derived insulin-like factor-3 (INSL3) acts on the oocyte to promote maturation. Members of the TGF-β family produced by granulosa cells play key roles in the local control of thecal androgen synthesis. BMP-6 suppresses basal and LH-induced thecal androgen secretion. GDF-9 (expressed by granulosa cells and the oocyte) inhibits human thecal androgen synthesis as well. TGF-β, which is produced by both granulosa cells and theca cells, reduces thecal androgen production. In addition, activin decreases thecal androgen synthesis, while inhibins increase it.
Theca cells of the ovulated follicle are incorporated into the corpus luteum, where they represent the population of small luteal cells (theca lutein cells), expressing the steroidogenic machinery to produce androgen precursors that are transformed by the larger luteal cells derived from the granulosa cells of the luteinizing follicle, which express aromatase activity.
Other Steroidogenic Cells in the Ovary
Hypertrophied theca interna cell remnants of atretic follicles are found in the ovary. These cells are thought to be targets of noradrenergic innervation that control steroidogenic activity. Hilar interstitial cells are large lutein-like cells with structural and functional characteristics indistinguishable from those of differentiated Leydig cells. Like Leydig cells, they contain the hexagonal Reinke crystals. Hilar cells are intimately associated with nonmyelinated sympathetic nerve fibers. The endocrine activity of these cells is assumed to be correlated with their prominence at the time of puberty, during pregnancy, and around the menopause.
Ovarian Stroma
The ovarian stroma contains fibroblastic cells that express some steroidogenic enzymes. The stroma may also harbor precursors of androgen-producing cells ( Fig. 8.12 ). Ovarian stroma cells express androgen receptors, and may proliferate under the influence of androgens, contributing to the increased stromal density characteristic of hyperandrogenemia of ovarian origin (e.g., PCOS and androgen-producing ovarian tumors).
The stromal cells serve as insulators in the ovary, separating follicles and corpora lutea from adjacent structures physically, as well as biochemically. Stromal cells produce growth factors and growth factor-binding proteins, the latter perhaps being key to the proposed insulator role. Ovarian stromal cells express substantial levels of gremlin, a protein that binds and inactivates BMPs; follistatin, which binds and inactivates activin; IGF-binding proteins (IGFBPs), and secreted frizzled-related proteins, which bind members of the WNT signaling family.
Ovarian Surface Epithelium
The ovarian surface epithelium, a flat to cuboidal epithelial layer of mesodermally derived cells, is also known as the ovarian mesothelium and by the misnomer of “germinal epithelium” because of the erroneous belief that it gave rise to germ cells. In the adult ovary, the surface epithelium is characterized by expression of the mucin gene, MUC1 , and the presence of cilia and apical microvilli. The cells sit on a basement membrane that covers a dense connective tissue layer.
The ovarian surface epithelium plays a role in the transport of material to and from the peritoneal cavity and in the repair of surface defects resulting from ovulation. During the ovulatory process, epithelial cells overlying the follicle undergo apoptotic cell death, followed by activation of a subsequent repair process. This process includes cell proliferation, which begins right after ovulation, and the resynthesis of extracellular matrix components. Proinflammatory cytokines may initiate these events.
Invaginations of surface epithelium into the ovary result in the formation of inclusion cysts. The ovarian surface epithelium in these inclusion cysts may undergo metaplasia, and possibly neoplasia. Although some researchers have suggested that these inclusion cysts form from invaginations around the site of ovulation, they have been reported to be prominent in PCOS, a condition characterized by oligoovulation or anovulation.
Ovarian Leukocytes and Macrophages
Macrophages, lymphocytes, and polymorphonuclear granulocytes are present in the ovary at various stages of its life cycle. These cells have roles in normal ovarian function as well as in ovarian pathology, where for example, lymphocyte infiltration, particularly in the theca interna, is a characteristic feature of autoimmune ovarian dysfunction.
Macrophages are a major cellular component of the ovarian interstitium, usually found near the perifollicular capillaries. Few other white blood cells are observed in the ovary in the early phases of follicular development, but a substantial infiltration of white blood cells occurs in the periovulatory period and in association with follicular atresia. Mast cells increase progressively in number during the latter portion of the follicular phase, and their release of histamine may contribute to ovarian hyperemia at ovulation.
After ovulation, eosinophils and T lymphocytes migrate into the corpus luteum, recruited by the expression of chemoattractants. The infiltration and subsequent activation of these cells occur before there is evidence of either functional or structural luteal regression. Activated T cells produce lymphokines that attract and activate macrophages. The dark stellate K cells that are scattered among the luteal cells are thought to be macrophages. They influence luteal cell function through discrete cell–cell contacts and the production of growth factors and cytokines. Regulatory T cells, a population of lymphocytes which maintain antigen-specific T cell tolerance, may be of particular importance. An abundance of these cells in the midcycle corpus luteum has been hypothesized to promote an antiinflammatory or “tolerant” state. Luteolysis is associated with a loss of regulatory T cells, which would engender a proinflammatory state. The importance of invasion of T lymphocyte subtypes and macrophages into the corpus luteum in the process of luteolysis is highlighted by the fact that pregnancy delays this invasion.
The neutrophils, eosinophils, lymphocytes, monocytes/macrophages, and mast cells in the ovary produce an array of cytokines including many members of the interleukin family, TNF-α, IFNγ, GM-CSF, and MIP-1α. These cytokines act locally and play significant roles in folliculogenesis as well as corpus luteum function.
Ovarian Innervation, Neurotrophins, and Tachykinins
The ovary has both extrinsic and intrinsic innervation. The extrinsic nerves, which are mainly sympathetic and sensory fibers with a small parasympathetic component, enter the ovary via the hilar perivascular plexus. The main function of this extrinsic innervation is to regulate ovarian blood flow. However, it may also affect pain in pathological states like ovarian endometriomas and the endocrine function of ovarian cells. Excessive activation of thecal adrenergic receptors has been proposed to play a role in the pathogenesis of ovarian hyperandrogenism. Consistent with this notion, histochemical evaluation of ovarian tissue obtained from patients with PCOS suggested enhanced innervation of the theca–interstitial cell compartment.
The human ovary intrinsic innervation includes neurons that are catecholaminergic, as identified by the expression of tyrosine hydroxylase, the rate-limiting enzyme in catecholamine synthesis. Most of these neurons express neurotrophin receptors.
The neurotrophins (NTs) are a family of nerve survival and differentiation factors. These molecules act on the TRK protooncogene family of high-affinity receptors and the low-affinity p75 nerve growth factor (NGF) receptor. NGF supports early follicular development. NGF-deficient mice, and mice lacking the NGF receptor, NTRK1, exhibit a substantial reduction in primordial follicle formation, and the ovaries have an increased number of oocytes that are not incorporated into a follicular structure. The absence of NTRK2, the receptor for neurotrophin-4 (NTN4), also known as NT-5, and brain-derived neurotrophic factor (BDNF), is also associated with reduced primordial follicle formation. Studies of knockout mice indicate that NTRK1 and NTRK2 receptors are required for assembly of primordial follicles and early follicular development, and that the action of NGF is in part mediated through the induction of FSH receptors in developing follicles. Mouse oocytes express the BDNF receptor TRK-B. BDNF, which is produced by granulosa cells in response to LH and NT-4/5, but not NT-3, stimulates mouse oocyte maturation, including extrusion of the first polar body, and promotes early embryonic development in vitro. Collectively, these findings suggest that the intraovarian neurotrophin system may be important for early follicular development and later oocyte maturation.
Neurotrophins and their receptors are also expressed in the human fetal ovary, with the TRK-B receptor being localized to germ cells and p75 NGF receptor in the stroma. BDNF, NT-4/5, and NT-3 are detectable in human follicular fluid aspirated from women undergoing controlled ovarian hyperstimulation.
In addition to neurotrophins and their receptors, the tachykinin system including substance P, hemokinin-1, the truncated receptor NTK1R-Tr, and NTK2R are expressed by human granulosa cells.
Ovarian Stem Cells
- ◆
There is a very small population of pluripotent stem cells in the adult ovary that can generate functional oocytes following manipulation in vitro if they are then transplanted into ovarian tissue.
- ◆
These rare stem cells do not appear to contribute significantly to the follicle pool under physiological conditions.
- ◆
Different types of human stem cells can be induced in vitro to develop into cells that closely resemble primordial germ cells.
Embryonic stem cells are pluripotent and have been shown to give rise to oocyte-like structures in vitro, raising the possibility of an ovarian stem cell population that could generate germ cells in adult life. Although this is a controversial notion, rare oogonial stem cells have been described in mouse and human ovaries, isolated through immuno-tagging with antibody to the N-terminus of DDX4, which detects a protein, probably not DDX4, that is expressed on the cell surface, and fluorescence-activated cell sorting. The specificity of DDX4 antibodies used in these studies has been questioned and the antigen they react with remains to be determined. The cells isolated with this potentially flawed methodology divide in culture and form oocyte-like structures based on their morphology and expression of oocyte-specific markers, including DDX4 and LHX8. These oocyte-like cells from mouse ovary develop in vitro and in vivo into oocytes that are capable of fertilization and embryonic development yielding live mice. Oogonial stem cells isolated from human ovarian cortex show similar characteristics. Oogonial stem cells transfected with a green fluorescent protein (GFP)-tagged lineage marker and injected into the ovaries of adult mice form GFP-expressing oocytes 5 to 6 months after injection. Some recovered GFP-tagged oocytes were successfully fertilized and subsequently developed in some instances to the blastocyst stage. Oogonial stem cells isolated from adult human ovarian cortical tissue and labeled with GFP were injected into human cortical strips that were transplanted into immunodeficient mice or re-aggregated with dissociated cells from cortical strips. Oocyte-like cells with meiotic markers and surrounded by somatic cells were found within grafts at 7 to 14 days after transplantation. Although the characterization of these oocyte-like cells was incomplete, these studies do provide evidence of a rare pluripotent stem cell population in the ovary. However, these cells do not appear to support follicular generation in numbers to prevent natural ovarian aging. Whether these stem cells make a quantitatively significant contribution to the ovarian follicular complement in normal women or women with ovarian dysfunction who have different follicular pool sizes (primary ovarian insufficiency or PCOS) remains to be determined.
Different types of stem cells can also be induced to develop into germ cell precursors. For example, human embryonic stem cells and induced pluripotent stem cells, if provided with the appropriate transcription factors and/or growth factors, will give rise to primordial germ cell-like cells in culture. These cells are currently being used for basic studies of the mechanisms of human germ cell specification and development, and there is optimism for their eventual future use in clinical settings.
Follicular Life Cycle
- ◆
The local communication among cellular components of the follicle modulates the actions of gonadotropins and the endocrine activity of the granulosa and theca cells, which in turn influences the pituitary output of FSH and LH and, consequently, the selection of a dominant follicle.
- ◆
The phosphoinositide 3-kinase, mTOR, and Hippo signaling pathways are critical regulators of the initiation of follicular growth.
- ◆
Follicular somatic cells (granulosa and theca cells) collaborate in the production of estrogens, and this collaboration continues into the luteal phase.
- ◆
Ovulation triggered by the LH surge requires the concerted action of granulosa cell products that regulate expansion of the cumulus, production of prostaglandins, and progesterone-dependent expression of key enzymes that promote follicular rupture.
- ◆
Corpus luteum function is dependent upon the action of LH and local luteotropic factors, including progesterone.
- ◆
The corpus luteum of the nonfertile cycle undergoes functional and then structural luteolysis, the former being driven by a decline in sensitivity to LH and a fall in steroidogenic acute regulatory protein, followed by apoptosis and autophagy of luteal cells.
- ◆
Local factors within the corpus luteum appear to regulate luteolysis.
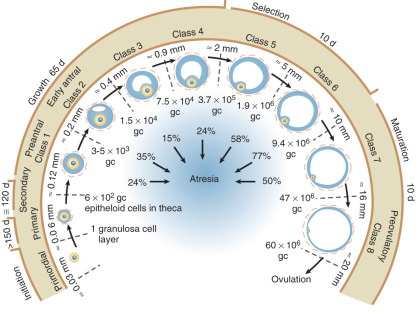
Gougeon proposed that the various follicular classes, defined largely by size and the number of granulosa cells, represent sequential stages of development on the way to maturity ( Fig. 8.13 ). According to this schema, approximately 1 year may elapse in the maturation of a primordial follicle to a dominant follicle. During much of this remarkably long period (approximately 300 days), follicles are believed to grow in a gonadotropin-independent manner. The gonadotropin-independent phase is driven by local factors acting in paracrine and autocrine dialogue between somatic cells and germ cells. Gonadotropins influence the last 50 days of the maturation process. They do so by activating signaling pathways that control gene transcription, posttranscriptional mechanisms including microRNAs, and posttranslational modification of proteins.
Follicular Growth
Follicular growth, which begins when primordial follicles emerge from their quiescent state, occurs continuously from the fifth to sixth month of intrauterine life until menopause. Although some researchers have suggested that the first formed follicles are the first ovulated, transition into the growth phase is more likely to be a random event independent of the sequence in which follicles formed during development.
The initiation of follicular growth is characterized by morphological changes, including a change in granulosa cell shape from flattened to cuboidal, proliferation of granulosa cells, enlargement of the oocyte, and formation of the ZP ( Fig. 8.14 ). The transformation of flattened granulosa cells to a cuboidal shape has functional correlates, including the expression of certain mRNAs (e.g., follistatin mRNA). Granulosa cell proliferation and the change to a cuboidal shape precede increases in oocyte diameter. In the human, the first substantial increase in oocyte diameter occurs when there are 15 granulosa cells in the largest follicle cross-section. Oocyte growth is accompanied by elaboration of the ZP, first evident as islands of periodic acid-Schiff-positive material. Subsequent increases in oocyte and follicular diameters are positively correlated until well into the secondary follicle stage, when oocytes reach a mean diameter of 80 µm. This stage corresponds to a follicular diameter of 110 to 120 µm and a granulosa cell endowment of approximately 600 cells. The germinal vesicle attains a mean maximal diameter of 26 to 27 µm at this follicular size.
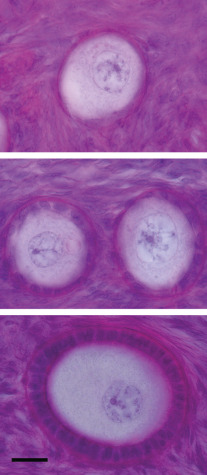
The early theca interna is acquired at the end of the primary follicle stage. The theca externa forms as the follicle expands and compresses the surrounding stroma (see Fig. 8.5 ). The migration of pretheca cells to the outer surface of the follicle is triggered by secreted signals from granulosa cells including Kit ligand and IGF-I. Signals secreted from the oocyte including FGF2, platelet derived growth factor (PDGF), GDF-9, and BMP-15 also promote theca cell migration and proliferation (see Fig. 8.7 ).
As a secondary follicle is being formed, the granulosa cells develop FSH, estrogen, and androgen receptors and become coupled by gap junctions. The formation of the thecal layer is associated with the development of a follicular blood supply from arterioles that terminate in a wreath-like network of capillaries adjacent to the basement membrane. Concomitantly, the theca cells acquire LH receptors and the capacity to synthesize steroid hormones. The secondary follicles constitute the pool of preantral follicles from which FSH-dependent recruitment of follicles takes place.
Oocyte Growth
A significant component of the secondary follicle growth phase is dedicated to oocyte differentiation and growth. The growing oocyte is a metabolically active cell, synthesizing mRNAs and proteins that support growth and development through the early preimplantation embryo stages. The oocyte is supported in its growth by bidirectional transport of nutrients, growth factors, and other molecules across the gap junctions that directly connect the transzonal projections of the surrounding granulosa cells to the oocyte.
Morphological alterations in the oocyte that occur during the growth phase include complete elaboration of the ZP by the active production, assembly, and secretion of its component proteins. The quantity of several cytoplasmic organelles increases, particularly the mitochondria, which are estimated to number approximately 400,000 in a fully grown human oocyte. In contrast, the centrioles that were present in oogonia are lost during the oocyte growth phase. The distribution of the organelles changes, with clustering of the mitochondria, endoplasmic reticulum, and Golgi complex in the region immediately surrounding the germinal vesicle.
During the growth phase, oocytes become competent to undergo meiotic maturation, or acquire “meiotic competence.” The molecular basis for the acquisition of meiotic competence is not completely understood. However, it occurs only after the oocyte has reached a critical size. Meiotically competent oocytes do have certain attributes, including increased levels of the cell cycle proteins CDK1, cyclin B, and CDC25. A threshold amount of these proteins is most likely a prerequisite for resumption of the cell cycle. Importantly, proper meiotic chromosome segregation depends on successful folliculogenesis and oocyte growth, processes that have substantial energy requirements. These requirements are met by transport of ATP and energy substrates from granulosa cells via gap junctions and also by oxidative phosphorylation of pyruvate within the oocyte proper. Mouse oocytes lacking PDH1A, an enzymatic subunit of pyruvate dehydrogenase, have decreased levels of ATP and nicotinamide adenine dinucleotide phosphate (NADPH) because they cannot perform oxidative phosphorylation of pyruvate. These oocytes grow and are ovulated, but do not successfully complete meiotic maturation. Similar processes may be compromised in the ovaries of older women, resulting in nondisjunction errors and aneuploidy in the embryo.
Although it is not completed until late in preimplantation embryo development, reestablishment of genomic imprints begins to occur during oocyte growth, at least partially due to the activity of DNA methyltransferases. Histone protein methylation also contributes to maternal imprint establishment as indicated by studies in mice lacking KDM1B, a histone H3 lysine 4 demethylase. Failure of the imprinting process in either oocytes or male germ cells results in aberrant expression of genes from the maternal or paternal alleles. These alterations in gene expression are associated with several inherited human diseases, including Beckwith-Wiedemann, Prader-Willi, and Angelman syndromes. A global loss of maternal imprinting in the oocyte results in the formation of a complete hydatidiform mole.
In addition to developing meiotic competence, oocytes in the growth phase begin to acquire the ability to support preimplantation embryo development and development to term, known as developmental competence . Functional aspects of developmental competence are poorly defined, but include the ability of the oocyte cytoplasm to remodel sperm DNA and an enhanced ability to generate calcium oscillations. Generation of a pool of maternal mRNAs that are highly stable but translationally repressed until maturation or fertilization is an important aspect of developmental competence.
At the completion of oocyte growth, transcription is actively silenced and protein translation slows substantially. Transcriptional silencing requires patent gap junctional communication with cumulus granulosa cells and is accompanied by changes in large-scale chromatin structure that are essential to confer growing oocytes with meiotic and developmental competence. Maintenance of these chromatin changes requires the activity of histone deacetylases. After transcriptional silencing, preovulatory oocytes rely on maternally derived proteins and mRNA stores to support the resumption of meiosis and the first cleavage divisions after fertilization.
Factors and Pathways Initiating Follicular Growth
Intraovarian factors are believed to play key roles in regulating the early phases of follicular growth either as activators or inhibitors. Activators of follicular growth and development include LIF, basic FGF, and Kit ligand. Kit ligand, which is produced by granulosa cells and acts on Kit, a receptor on the oocyte and theca cells, is required for initiation of follicular growth and growth of the oocyte. An antibody to Kit that blocks interaction with Kit ligand interferes with the primordial stage of follicle development when injected into newborn mice. Conversely, recombinant Kit ligand, both the diffusible and membrane-bound isoforms, accelerates the transition from primordial to primary follicles in neonatal rat ovaries. Anti-müllerian hormone, activin A, and the chemokine SDF-1/CXCL12, acting through its receptor, CXCR4, inhibit follicular growth.
The oocyte-derived proteins GDF-9 and BMP-15 are important for granulosa cell proliferation in a species-specific manner, as shown by the ovarian phenotypes of the Gdf9 knockout mouse and sheep with homozygous mutations in the Gdf9 and Bmp15 genes. With GDF-9 deficiency, granulosa cells cease proliferating after approximately two doublings. The oocytes continue to grow, however, resulting in large oocytes that ultimately degenerate and are surrounded by a single layer of granulosa cells.
Two signal transduction cascades play critical roles in the activation of primordial follicles: the phosphatidylinositol-3-kinase (PI3 kinase)/Akt pathway and the mTOR pathway. When disrupted, both pathways result in aberrant activation of follicular growth in mice and the ultimate depletion of primordial follicles.
PI3 kinase catalyzes the formation of phospholipid, 3,4,5 phosphoinositol phosphate (PIP3), which activates protein kinase PDK1, which in turn phosphorylates the kinase Akt, which phosphorylates the forkhead O3 transcription factor, FOXO3. Kit ligand, produced by granulosa cells, acts on Kit expressed on the oocyte and appears to activate this cascade, which ultimately leads to phosphorylation and inactivation (exclusion from the nucleus) of FOXO3, resulting in the inhibition of genes required for the initiation of follicular growth. Mice with null mutations for Foxo3 do not restrain primordial follicle activation, which results in global follicle activation soon after birth and subsequent premature primordial follicle depletion and sterility. The regulatory components involved in FOXO3 phosphorylation include PDK1 and PTEN (phosphatase and tensin homologue deleted on chromosome 10). Oocyte-specific deletion of Pdk1 causes infertility secondary to depletion of primordial follicles. PTEN, is a PIP3 phosphatase and, therefore a negative regulator of phosphatidylinositol 3-kinase. Deletion of PTEN in the oocyte, which removes a restraint on activation of PDK1 and Akt, also results in activation of primordial follicular growth and early depletion of follicles.
mTOR is a member of the phosphatidylinositol-3-kinase family. mTOR complex 1 (mTORC1), one of two mTOR complexes with distinct functions, regulates transcription, translation, and metabolism through downstream targets. It is negatively regulated by a complex formed by two unrelated proteins, tuberous sclerosis complex (TSC)1/TSC2, that work through the intermediacy of a small GTP hydrolase, RHEB, which activates mTORC1 that in turn activates translation by phosphorylating ribosomal S6 kinase and 4E-binding protein 1. When Tsc1 and Tsc2 function is ablated in mouse oocytes, the primordial follicle pool is established, but premature follicular activation depletes this pool resulting in infertility. The follicular activation in mice lacking TSC1 can be suppressed by the administration of the mTOR inhibitor, rapamycin. The serine/threonine kinase LKB1 is required to restrain primordial follicle activation via suppression of the mTOR pathway. Although these observations provide strong evidence for the importance of the mTOR signaling pathway in follicular growth control, the upstream factor that initiates the signal transduction cascade has not been elucidated.
In addition to the PI3 kinase and mTOR pathways, several transcription factors are known to play roles in the transition from primordial to primary follicles based on functional genomic studies in mice, including NOBOX, SOHLH1, and SOHLH2. Mice with mutations in Nobox , Sohlh1 , or Sohlh2 have defects in the primordial to primary follicle transition and are infertile. Similarly, the cell cycle regulator CDKN1B functions in the nucleus of primordial oocytes to restrain premature follicle activation.
The Hippo signaling pathway plays a key role in determining organ size and tissue homeostasis. In its active state, it suppresses growth. The signaling pathway involves a cascade of protein kinases including MST1 and MST2, which phosphorylate the adaptor protein SAV, which forms a complex that phosphorylates LATS1 and LATS2, which associates with the adaptor proteins MOB1A/B and phosphorylates the transcriptional coactivators YAP1 and TAZ. Phosphorylated YAP1/TAZ are retained in the cytoplasm. Inactivation of Hippo signaling leads to hypophosphorylation of YAP1 and TAZ, followed by their translocation to the nucleus where, in conjunction with binding partners such as TEAD1-4, they alter transcription, resulting in cellular proliferation and/or survival. This pathway has a potential role in follicular development in concert with the Akt and mTOR pathways.
Hippo pathway transcripts and proteins are present in the ovary, and they decline as follicles progress from the primordial to the antral stage. Ovarian fragmentation appears to lead to inactivation of Hippo signaling with subsequent growth of follicles towards the antral stage in the presence of Akt activation. The importance of genes encoding Hippo pathway components in ovarian function has been revealed in mouse knockout models, which exhibit fertility defects and abnormal ovarian morphology including the development of ovarian cysts. Interestingly, genome-wide association studies identified the locus encoding YAP1 as a PCOS candidate gene.
Pharmacological manipulation of the Akt, mTOR, and Hippo pathways using PTEN inhibitors, PI3K stimulators, or mTOR activators has been proposed as treatment for premature ovarian insufficiency. Proof-of-principle studies on mouse and human ovarian tissue have been published and live births from IVF procedures on women with premature ovarian insufficiency whose ovarian tissue was treated in vitro with drugs that impact these pathways have been reported. This technology has been referred to as in vitro activation.
Evidence suggesting that FSH is not required for initiation of follicular growth includes the fact that the process occurs in hypophysectomized animals. In humans and mice with inactivating mutations in the FSHβ subunit or FSH receptor gene, follicular development can occur to the secondary and early antral stages, but more slowly and with markedly reduced frequency than when normal levels of FSH activity are present. However, the importance of pituitary-derived factors (not necessarily FSH) in follicular growth and survival in the primate fetus was illustrated by the observation that hypophysectomy of the fetal Rhesus monkey results in oocyte depletion. Moreover, studies on rodent ovaries suggest that preantral follicles are gonadotropin-responsive. In human ovarian xenografts transplanted to immunodeficient and hypogonadal mice, FSH was shown to be required for growth of follicles beyond the two-granulosa-layer stage. Thus follicle growth before the antral stage, although possible in the absence of gonadotropins, may be facilitated by FSH.
Formation of Antral Follicles
There is little doubt that the transition from the secondary-follicle to the antral-follicle stage is promoted by FSH. Antral follicles are rarely observed in animals or humans with FSH deficiency (unless exogenous FSH is administered) or ovaries lacking FSH receptors. The antrum and its fluid facilitate the process of release of the cumulus–oocyte complex at ovulation and serve as a vehicle for nutrient exchange and waste removal in the avascular compartment. The antrum also serves as a unique environment in which the cumulus–oocyte complex completes growth and maturation.
The development of the antrum requires the influx of water, which occurs primarily through a transcellular process. This may be mediated by the water channels formed by aquaporins 7, 8, and 9. Because net transfer of water via the aquaporins requires an osmotic gradient, granulosa cells are believed to actively transport ions to create this gradient. Alternately, the hydrolysis of glycosaminoglycans in the antrum could increase the osmolarity of follicular fluid and support the influx of water.
Between 5 and 6 days before ovulation, the follicle undergoes rapid expansion—as a result of granulosa cell proliferation and the accumulation of antral fluid—and moves to the ovarian surface. The accelerated expansion of the follicle destined to ovulate can cause midcycle pelvic pain (mittelschmerz). The expression of the cell cycle gene, cyclin D2, is important for this expansion, because cyclin D2 null mice show impaired granulosa cell proliferation and consequently have an ovulation defect. On completion of this growth phase, the follicle, now referred to as a Graafian follicle, is prepared for ovulation.
Follicular Recruitment, Selection, and Dominance
The term recruitment has been used to describe the process by which the follicle departs from the resting pool to initiate growth. However, some authors also use this term to describe the engagement of a cohort of antral follicles into further growth. It has been suggested that the first situation be called initial recruitment and the latter, cyclic recruitment. Cyclic recruitment, although obligatory, does not guarantee ovulation because growing follicles are vulnerable to atresia and thus may fall out from the growth trajectory. Selection refers to the process by which the maturing follicular cohort is reduced to a number appropriate for the species-specific ovulatory quota. This process entails negative selection against the subordinate follicles as well as positive selection of the follicles that will determine dominance.
Although traditional thinking holds that a single cohort (wave) of follicles develop during the menstrual cycle, ultrasound studies suggest that multiple waves occur. The “wave theory,” supported by serial transvaginal ultrasound monitoring of normal ovulatory women and some earlier histological studies of follicular recruitment, holds that two or more cohorts of 4 to 14 follicles greater than or equal to 4 to 5 mm in diameter are recruited during the ovarian cycle, and that the dominant follicle that emerges in the final wave of the interovulatory interval is destined to ovulate.
In the early follicular phase, no gross morphological differences exist between the selected follicle and other healthy members of the cohort. However, the leading follicle can be distinguished from other members of the cohort by its size and the high mitotic index of its granulosa cells. Only the leading follicle has detectable levels of FSH in its follicular fluid. The leading follicle also contains significant levels of estradiol, a hallmark of the chosen follicle. Selection does not guarantee progression to ovulation, but given its temporal proximity to this event, ovulation usually does occur.
FSH is required for the transition of secondary preantral follicles to the antral stage. It also is a survival factor for antral follicles, and its withdrawal triggers programmed cell death in the absence of local factors that sensitize the follicle to FSH action or amplify its effects. Follicular maturation initiated at the start of a new menstrual cycle is driven by an increase in FSH levels in the late luteal phase as progesterone, estradiol, and inhibin A levels fall. Preantral follicles apparently require a threshold FSH concentration to sustain growth, and this threshold level is reached during the late luteal phase. Remarkably, the threshold can be crossed with as little as a 10% to 30% increment in FSH, indicating that granulosa cells have a highly sensitive, but still mysterious, detection system with which they interpret circulating FSH levels. FSH can induce follicular growth to the preovulatory size of at least 17 mm in the virtual absence of LH. Although estradiol production is impaired under these circumstances, inhibin production is induced-reflecting the normal response of granulosa cells to FSH.
Granulosa cell division is promoted by FSH, possibly by an indirect mechanism. This action is mediated by growth factors produced by either somatic cells or the oocyte. For example, in rodents, estrogens produced in response to FSH are an important mitogen for granulosa cells. An early sign that a dominant follicle has been selected is that its granulosa cells proliferate at a greater rate than the cells in the nondominant follicles. The differential mitotic rates can be detected in the late follicular phase.
One of the major actions of FSH is the induction of aromatase in granulosa cells (see Fig. 8.8 ). Thus little or no estrogen can be produced by FSH-unprimed granulosa cells, even if they are supplied with aromatizable androgen precursors. FSH also induces expression of cytochrome P450 reductase, which transfers electrons to aromatase, and type 1 17β-hydroxysteroid dehydrogenase (17BHSD1), the “estrogenic” 17β-hydroxysteroid dehydrogenase that reduces estrone to estradiol.
Follicle-stimulating hormone induces LH receptors in the granulosa cells of the preovulatory follicle. LHR mRNA is detectable in antral follicles (3 to 10 mm in diameter) and reaches maximal levels in granulosa cells in preovulatory follicles. In contrast, FSHR mRNA levels decline in granulosa cells as follicular diameter increases. Consequently, in the late stages of follicular maturation, LH can subserve FSH’s function in propelling follicular maturation. This attribute may allow the dominant follicle to complete its maturation cycle in the face of declining FSH levels; in addition, the dominant follicle is prepared to respond to the ovulatory LH surge.
The importance of FSH in follicular development has been documented by the discovery of mutations that inactivate the FSH β subunit and the FSH receptor in humans, and by targeted deletions of these genes in mice. Women who are homozygous for FSH receptor mutations have the features of hypergonadotropic hypogonadism, with absent or poor development of secondary sexual characteristics and high FSH and LH levels. The ovarian phenotypes of humans with these mutations, and those of FSH receptor and FSH β subunit knockout mice are similar. In the absence of functional FSH β subunit or FSH receptor, the ovaries are small and follicular development generally goes no further than the preantral stage.
Dominance refers to the status of the follicle destined to ovulate, and its role in regulating the size of the ovulatory quota. The follicle destined to ovulate attains dominance 5 to 7 days after the demise of the corpus luteum of the previous cycle. This conclusion is supported by the observation that the levels of estradiol in the ovarian vein are remarkably different between ovaries by day 5 to day 7 of the cycle, attesting to the emergence of the dominant follicle. This follicle continues to thrive under circumstances that it has made inhospitable for competing follicles in both ovaries.
The control of the temporal sequence of events leading up to follicular dominance has been elucidated by ablation studies in infrahuman primates, and also in women in which the dominant follicle or corpus luteum was destroyed or removed. Destruction of the largest follicle on day 8 to day 12 in the primate ovary delays the next preovulatory surge of pituitary gonadotropins. Conversely, luteectomy in the midluteal phase (days 16 to 19) advances the gonadotropin surge. In women, the interval from ablation of the dominant follicle or corpus luteum to the next ovulation is 14 days. These findings are consistent with the notion that the cyclic structures of the dominant ovary (i.e., the ovary containing the dominant follicle or corpus luteum) are the timekeepers of the menstrual cycle. The 28-day menstrual cycle is thus the result of the intrinsic lifespan of the dominant follicle (follicular phase) and corpus luteum (luteal phase), not timing dictated by the brain or pituitary. These elegant experiments were conducted prior to the use of ultrasound monitoring of follicular growth, so it is not possible to reconcile these observations with sequential ultrasound studies of follicular dynamics in cycling women (i.e., follicular waves).
The selection of the follicle destined to ovulate has already occurred early in the cycle. No other member of the follicular cohort is competent to serve as a surrogate for a destroyed dominant follicle, and a timely midcycle gonadotropin surge is not achieved. In the case of the corpus luteum, the next round of follicular growth occurs only after its interference is removed—either naturally (luteolysis) or artificially (luteectomy). Studies on hormone-replaced luteectomized primates suggest that progesterone is the principal agent responsible for the inhibition of follicular growth in the luteal phase. However, inhibin A secreted by the corpus luteum may also play a role in the suppression of FSH and thus follicular maturation. Also central to the process of follicular development is its vasculature. Inhibition of the action of vascular endothelial growth factor (VEGF) blocks follicular maturation secondary to an attenuation of follicular vascular density or reduced vascular permeability, which may limit access of critical growth factors or hormones necessary for follicular growth.
Endocrine Characteristics of Follicles on the Way to Dominance
Follicles with a diameter of less than 8 mm have a relatively low intrafollicular estrogen-to-androgen ratio, but from the midfollicular phase onward, this ratio is reversed ( Fig. 8.15 ). The “chosen” follicle is able to synthesize estradiol in sufficient quantities to result in appreciable passage of this hormone into the general circulation, and asymmetry in ovarian estrogen secretion as early as day 5 to day 7 of the cycle. In the late follicular phase, the intrafollicular concentrations of estradiol are directly correlated with follicular size and achieve concentrations of approximately 1 µg/mL at a time when circulating estradiol levels reach their peak. After the ovulatory surge of LH, intrafollicular concentrations of estradiol decline and there is a parallel decrease in the concentration of androstenedione. Concurrently, progesterone and 17α-hydroxyprogesterone concentrations increase, reflecting early granulosa cell luteinization.
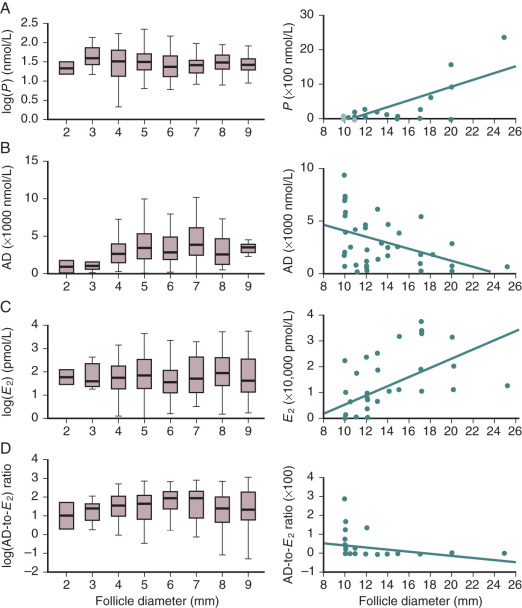
Inhibin A concentrations in follicular fluid increase with follicular maturation, whereas inhibin B, activin A, and free follistatin do not show variations with follicular size. Thus as follicles mature, there is a switch from an environment dominated by activin to one dominated by inhibin A. The increase in inhibin A levels is correlated with increased expression of inhibin A α and β subunit mRNAs in granulosa cells.
The IGFs are members of a family of low-molecular-weight, single-chain polypeptide growth factors named for their structural and functional similarity to insulin ( Fig. 8.16 ). Both IGF-1 and IGF-2 are present in human follicular fluid. Follicular fluid IGF-1 is most likely derived predominantly from plasma. IGF-2, however, is produced by the theca and perifollicular vessels of all follicles, and the granulosa and theca cells of small antral follicles, and is abundantly expressed by preovulatory granulosa cells. In mice lacking IGF-1, follicular maturation is arrested; in addition, the animals are infertile, and granulosa cell proliferation in the basal state and in response to estrogen is impaired. In women with Laron dwarfism, a disease characterized by IGF-1 deficiency, ovulation induction with human menopausal gonadotropins after administration of a GnRH analog resulted in development of mature follicles and fertilizable oocytes. This finding indicates that, in the human, IGF-1 is not essential for normal follicular development. IGF-2, however, would be present in this situation. Infusion of IGF-1 at levels that raise blood concentrations twofold above normal have no effect on ovarian function in Rhesus monkeys. Thus the locally produced IGFs appear to be sufficient to promote normal ovarian activity, and elevated IGF levels do not disrupt ovarian function.
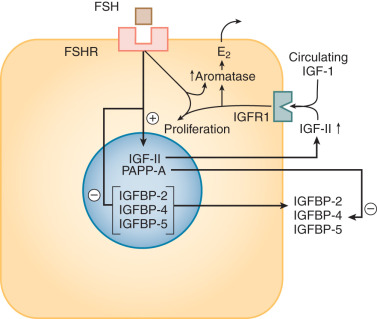
In situ hybridization studies showed that IGF-1 receptors, which are activated by both IGF-1 and IGF-2, are present in the granulosa cells of dominant follicles. IGF-2 receptors, which probably are not involved in signaling, are found in theca and granulosa cells. IGF-1 and IGF-2 stimulate DNA synthesis, granulosa cell proliferation, and steroidogenesis by cultured human granulosa and granulosa–lutein cells. However, these in vitro experiments were conducted in restricted culture medium, so the addition of general trophic factors might be expected to increase cellular function.
The action of IGFs is modulated by the local elaboration of binding proteins. Of the seven IGFBPs that have been described to date, at least five are expressed in the human ovary. The IGFBPs bind IGFs and neutralize their activity, and they also may have direct actions on ovarian cells. IGFBP-1, -2, -3, -4, and -5 have been identified either in follicular fluid or by analysis of mRNA from granulosa cells. Of these binding proteins, IGFBP-4 is of particular interest because it is a potent antagonist of FSH-stimulated estradiol production by human granulosa cells. IGFBP-4 also is present in atretic follicles, implicating this protein in the pathway leading to follicular atresia.
Secretion of IGFBPs is inhibited by gonadotropins and IGFs, resulting in enhanced IGF bioavailability and gonadotropin action. The proteolytic cleavage of IGFBPs is another mechanism controlling IGF bioavailability. IGFBP-4 protease expression is restricted to healthy follicles and corpora lutea. The metalloproteinase activity of pregnancy-associated plasma protein A (PAPP-A), a large dimeric glycoprotein, degrades IGFBP-4 into inactive fragments. Granulosa cells from small follicles secrete low levels of PAPP-A, whereas granulosa cells from dominant follicles secrete high levels of this protein. The significance of this proteolytic mechanism is underscored by the fact that mice lacking PAPP-A have a reduced number of ovulated oocytes in a stimulated ovulation protocol, which is associated with lower serum estradiol levels, lower serum progesterone levels, and reduced expression of ovarian steroidogenic enzyme genes.
Insight into the importance of estrogens in ovarian function in women comes from the study of subjects in whom estrogen synthesis is impaired. Limited studies have been performed on women with 17α-hydroxylase/17,20 lyase (CYP17A1) deficiency who are incapable of producing thecal androgens to support granulosa cell estradiol synthesis. Promotion of follicular growth to the preovulatory stage in an estrogen-impoverished environment is possible in these individuals with exogenous gonadotropins after pituitary desensitization. The same is true in severely hypogonadotropic women given exogenous FSH. Follicles grow, but in the absence of exogenous LH, estradiol synthesis is minimal. Moreover, the development of follicular cysts with low estrogen levels is common in women with steroidogenic acute regulatory protein (STARD1), 17α-hydroxylase/17,20 lyase, and aromatase deficiencies. Hence the high levels of estrogen associated with normal follicular maturation are not required for the growth of follicles to the size equivalent to the preovulatory stage.
Whether oocytes that are recovered from estrogen-deprived follicles are endowed with the properties that can lead to successful embryonic development after fertilization is less certain based on limited experience in humans with defects in ovarian estrogen biosynthesis. Animal studies with aromatase inhibitors suggest that estrogens are important for oocyte function. Rhesus monkeys treated during follicular maturation with doses of an aromatase inhibitor that substantially reduces circulating estradiol levels showed no effects on follicular growth. However, a greater proportion of the oocytes recovered from follicles of the aromatase-treated animals were in prophase I, and there was retarded completion of maturation to MII. Whether this is a direct reflection of estradiol deficiency, a consequence of the suicide aromatase inhibitor (1,4,6-androstatrien-3,17-dione), or the result of compensatory changes in endocrine status due to the decline in estradiol is not known. However, clinical experience with competitive aromatase inhibitors like letrozole indicates that aromatase inhibition during follicular growth does not have a major negative impact on oocyte maturation.
The human ovary expresses the receptors that allow a variety of cells to respond to estradiol, but the physiological roles of estrogen in follicular health, maturation, and luteal function remain to be elucidated. Although estrogen receptor α and β1 and β2 are expressed, with the latter two receptor types being more prominent in luteinized granulosa cells, the extremely high levels of estradiol reached in the antrum of the preovulatory follicle (approximately 1 µg/mL) raise questions as to the function of the classical estrogen receptor system, which would be fully saturated by ligand. Moreover, follicle growth per se does not require high levels of estradiol as noted above.
Androgens have a number of effects on the primate ovary. Administration of testosterone or 5α-dihydrotestosterone to Rhesus monkeys promotes accumulation of primary follicles as well as follicle survival, suggesting a folliculotropic action. In this model, androgen receptors are abundant in the granulosa cells of healthy preantral and antral follicles, with lesser expression in the theca and stroma. Moreover, androgen receptors were positively correlated with a marker of cell proliferation (Ki-67) and negatively correlated with apoptosis. Supplementation with androgens has been proposed by some investigators as a treatment for poor responders to gonadotropins. However, it is unlikely that any beneficial effect of supplementation is due to the direct action of dehydroepiandrosterone or its androgenic metabolites on the ovary. These observations contrast with the view that androgens are atretogenic, a concept that emerged primarily from studies on the rodent ovary in which androgens block granulosa cell proliferation in vitro in some systems and promote follicular atresia.
Evidence that androgens have a detrimental effect on human follicular function includes the observation that follicular fluid enriched in 5α-dihydrotestosterone and poor in estradiol is characteristic of atresia. However, this steroid profile may be a consequence rather than a cause of atresia. Favoring a causal relationship are reports that high follicular concentrations of 5α-reduced androgens, such as 5α-dihydrotestosterone, act as competitive inhibitors of granulosa cell aromatase activity. In this regard, follicles from patients with PCOS have greater 5α-reductase activity than follicles from normal ovaries. Thus androgens may exert both positive and negative effects on follicular growth and function in a stage-dependent manner through androgen receptors as well as by nonreceptor-mediated mechanisms.
Ovulation
As midcycle approaches, the rise in estrogen emanating from the dominant follicle initiates an LH surge and, to a lesser extent, an FSH surge. This triggers the resumption of meiosis, ovulation, and luteinization ( Fig. 8.17 ). Somatic ovarian cells, leukocytes, and macrophages, through release of matrix degrading enzymes and cytokines including interleukin (IL)-1β and TNF-α, are thought to play roles in this process.

In a normal menstrual cycle, the FSH-induced appearance of LH receptors on preovulatory granulosa cells allows LH to take over FSH’s functions in the terminal stages of follicular maturation. These receptors also enable the granulosa cells to become competent to respond to the LH surge that initiates the resumption of meiosis, ovulation, and subsequent luteinization of the granulosa and theca cells. These events are triggered only when a threshold concentration of LH is achieved. Notably, granulosa cells respond to FSH with activation of adenylate cyclase, but genes or events that constitute the program for ovulation and luteinization are not induced. The necessity for some LH to stimulate thecal androgen production and synergize with FSH for follicular maturation, together with the potential for high levels of LH to promote premature luteinization and possibly atresia of follicles that have not reached the Graafian stage, has led to the notion of an “LH window” for follicular maturation. This concept has pharmacological and clinical relevance with respect to ovulation induction. Levels of LH that are capable of stimulating maturation of the dominant follicle retard the growth of smaller follicles and suppress aromatase activity. This effect has led to the theoretical possibility of minimizing multifollicular development by using LH or hCG to drive terminal stages of follicular maturation.
The threshold for activation of the ovulation–luteinization program may be sensed by granulosa and theca cells as a result of the intensity of the signal (i.e., the magnitude of the increase in cAMP); it may also be sensed by the activation of adjunctive signal transduction cascades that supplement the increase in cAMP. LH receptor activation of cAMP and IP3 signaling are dependent on LH dose, with a 10- to 100-fold higher level of LH needed to activate phospholipase C. These pathways influence expression of noncoding RNAs, which appear to play a key role in coordinating gene expression patterns in the development of the ovulatory follicle and the response to the ovulatory LH surge.
LH initiates the sequence of events resulting in ovulation in part through the intermediacy of mitogen-activated protein kinases (MAPK). Studies in mouse double mutants for Mapk1 and Mapk3 revealed a phenotype of sterility associated with defects in cumulus expansion, ovulation, luteinization, and meiotic maturation. Moreover, granulosa cells continued to proliferate and aromatase expression was maintained, indicating that the differentiation process triggered in granulosa cells by the LH surge involves the MAPK signaling pathway.
LH receptor knockout mice have a relatively normal theca layer around developing follicles. However, follicular maturation is arrested at the early antral stage, and there are no signs of ovulation or luteinization. This phenotype is similar to that found in women with homozygous inactivating mutations in the LH receptor gene (LHCGR) . The clinical phenotype of affected women includes normal primary and secondary sexual characteristics, amenorrhea, and elevated circulating FSH and LH. The ovaries contain follicles in stages of development from primordial to antral, with well-developed theca layers, but no preovulatory follicles or corpora lutea. One homozygous misssense LHCGR variant (N400S) was associated with empty follicle syndrome. The phenotypes described to date support the notion that LH is required for normal estrogen production by follicles, for ovulation, and for luteinization, but not for the formation of the theca layer.
Activating mutations of the human LH receptor are also informative regarding the role of LH in ovarian function. Women with these mutations have no obvious reproductive phenotype, in contrast to the precocious puberty found in males. One might have anticipated a hyperandrogenemic state in females, mimicking PCOS with thecal hyperplasia. However, follicular compartments evidently develop in a coordinated fashion such that thecal responses to LH receptor activation do not occur prematurely. Moreover, levels of cAMP or other second-messenger molecules required for luteinization must be different from those generated by the constitutively active mutant receptor because premature luteinization of follicles does not occur.
The preovulatory LH surge precedes follicular rupture by as much as 38 hours. Before rupture, a number of critical changes take place in the granulosa cells and oocytes, including the suppression of transcription of genes that control granulosa cell proliferation; the loss of gap junction function, which uncouples the electrophysiological syncytium of the granulosa cells and the oocyte; and the induction of genes essential for ovulation in granulosa cells, including the genes encoding the EGF-like factors amphiregulin, epiregulin, and betacellulin ( Fig. 8.18 ). In mice, the latter growth factors activate EGF receptors, resulting in the induction of the gene encoding cyclooxygenase-2 (COX-2; also known as PTGS2) in granulosa cells and, consequently, prostaglandin E 2 (PGE 2 ) synthesis. PGE 2 acts in concert with the EGF-like factors to trigger the cumulus cells to elaborate a hyaluronan-rich matrix that causes expansion of the cumulus. As described later, and in Chapter 4 , mice deficient in PTGS2 and the prostaglandin receptor EP2 (PTGER2) have ovulation defects that are related to an abnormality in cumulus expansion.