Ovarian and Fallopian Tube Cancer
Ovarian neoplasms encompass a wide array of benign and malignant tumors with diverse histologic cell types, clinical features, and survival outcomes. Primary malignant tumors of the ovary include the epithelial ovarian cancers, germ cell tumors, and sex cord tumors. Low malignant potential (LMP) tumors of the ovary are noninvasive epithelial tumors often confined to the ovary, although extraovarian tumor implants may be detected. Metastases to the ovary occur from other primary malignancies including uterine, gastrointestinal (Krukenberg tumors), and breast cancers. Primary lymphoma, sarcoma, and melanoma of the ovary are rare. Relative to its incidence, epithelial ovarian cancers have substantially high mortality because effective screening tools are lacking; only 25% are detected as stage I at diagnosis, and current therapies for advanced cancer, although improving, have reached a therapeutic plateau. Surgery is the mainstay for diagnosis, staging, and the initial treatment for ovarian cancer. Platinum-based chemotherapy is indicated for patients with high-risk or advanced disease. Novel agents, such as antiangiogenics and poly (ADP-ribose) polymerase (PARP) inhibitors, are under active investigation in the adjuvant and/or recurrent setting. The use of whole-abdomen irradiation (WAI) or intraperitoneal (IP) radioisotopes is primarily historical, and radiation therapy now has a limited role in the management of ovarian cancer. Nonetheless, palliative radiotherapy may be of significant benefit for symptomatic disease relapse or select patients with localized recurrence.
 ANATOMY
ANATOMY
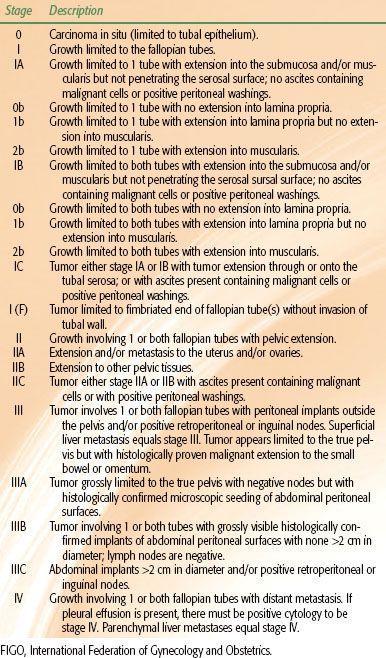
In premenopausal women, the ovaries are almond-shaped, gray-pink solid organs that measure approximately 4 é 2.5 é 1 cm, with an average weight of 4 to 5 g. After menopause, the ovaries atrophy and become nonfunctional and smaller in size. When normally positioned, the ovary is attached by the meso-ovarium to the broad ligament that covers the uterus and fallopian tubes. The infundibular pelvic, or suspensory, ligament extends from the surface of the ovary to the lateral pelvic wall, forming the superior and lateral aspect of the broad ligament (Fig. 71.1). The blood supply of the ovary is derived from the ovarian arteries, which arise from the aorta immediately below the level of the renal arteries and course through the retroperitoneum and infundibular pelvic ligaments. The venous return of the ovary empties to the renal vein on the left and directly to the vena cava on the right. The primary lymphatic drainage of the ovary parallels the course of the ovarian veins, with secondary lymphatic flow passing through the inguinal canal and to the iliac nodal system.1 Histologically, the outer cortex of the ovary is covered by a layer of pseudocolumnar or cuboidal epithelium, termed the germinal epithelium of Waldeyer or ovarian surface epithelium (OSE). The inner medulla consists of a superficial tunica albuginea and dense stromal tissue filled with blood vessels and spindled, “muscle-like” connective tissue. Within the medulla, maturing follicles are present throughout the various layers.
The fallopian tubes are positioned horizontally within the superior part of the broad ligament and extend from the superior posterior portion of the uterine fundus to the ovaries. The fallopian tubes are hollow, muscular viscera that are in direct communication with the peritoneal cavity. The ovarian artery anastomoses with the uterine artery to supply the fallopian tube, and venous drainage is through the pampiniform plexus to the ovarian vein and uterine plexus. The mucosa of the fallopian tube contains a rich network of intercommunicating lymphatic sinusoids that anastomose with adjacent organs and drain into the ovarian lymphatics and para-aortic and iliac lymph nodes.2 The fallopian tubes consist of four separate histologic layers: the mucosa, submucosa, muscularis (external longitudinal and inner circular layers), and outer serosal layer, which is continuous with the visceral peritoneum of the uterus. The mucosa is intricately folded, with the number of folds increasing from the interstitial portion to the ampulla. The epithelium is composed mainly of ciliated cells and secretory cells. Cyclic changes are evident in the tubal epithelium, similar to those of the endometrium, in response to estrogen and progesterone.
FIGURE 71.1. Anatomy of the ovary and female reproductive tract. (Asset provided by the Anatomical Chart Company.)
 EPIDEMIOLOGY
EPIDEMIOLOGY
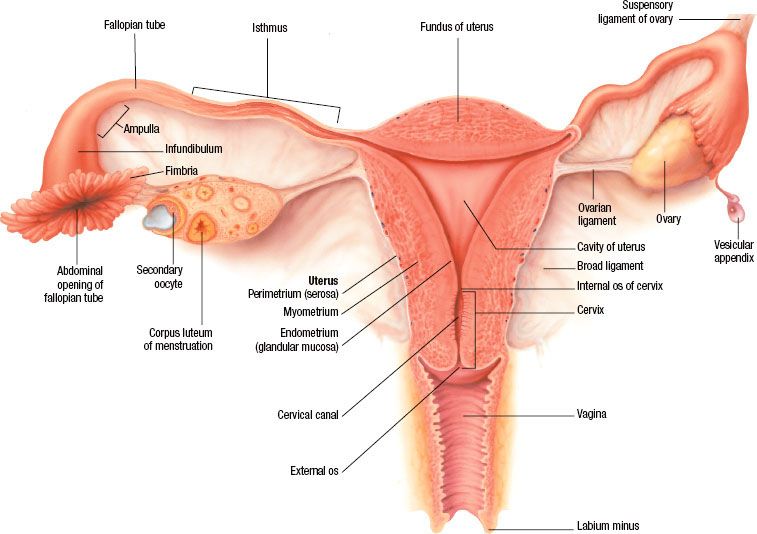
Ovarian cancer is the second most common gynecologic malignancy in the United States after endometrial cancer. Approximately 22,280 women in the U.S. received a diagnosis of epithelial ovarian cancer in 2012, and 15,500 died of the disease3,4 (Fig. 71.2). Ovarian cancer represents the fifth leading cause of cancer-related death in U.S. women, following lung, breast, colorectal and pancreatic cancers. The lifetime risk of ovarian cancer is approximately 1 in 72 women with a median age at diagnosis of 63 years; >80% of women are diagnosed after the age of 40 years.5 The incidence of ovarian cancer rises with increasing age and peaks in the eighth decade of life. Differences in race and ethnicity are apparent in the age-adjusted annual incidence per 100,000 women, which in 2005 to 2009 was highest for White women (13.4), followed by Hispanic (11.3), American Indian/Alaska Native (11.2), Black (9.8), and Asian/Pacific Islanders (9.8).5 The incidence of ovarian cancer also varies significantly by geography, with the highest rates in North America and northern Europe, which are three to seven times higher than that observed in Japan.6 Mortality rates for ovarian cancer have been decreasing in the United States since 1975, when the 5-year survival rate reported by the Surveillance, Epidemiology, and End Results (SEER) Program was 37%. Between 2001 and 2007, 5-year survival increased to 44%, predominantly driven by survival gains among White women. Black women have disproportionately lower 5-year survival rates, estimated at 34% in the same time period.5
Primary cancer of the fallopian tube was once considered a rare disease, accounting for 0.2% to 0.5% of female gynecologic malignancies. However, the true incidence has likely been underestimated considering a large proportion of extrauterine high-grade serous carcinomas may actually originate in the fimbriated end of the fallopian tube.7,8 Precursor lesions, known as tubal intraepithelial carcinomas (TICs) have been detected in the fallopian tubes of prophylactic salpingo-oophorectomy specimens from high-risk patients. The model of the fallopian tube as the primary site of origin for extrauterine high-grade serous carcinoma is supported by epidemiologic, morphologic, and genetic data, as discussed later.
 PATHOGENESIS
PATHOGENESIS
The female reproductive tract originates from the Müllerian ducts, which are paired embryologic structures of mesodermal origin that give rise to the fallopian tubes, uterus, cervix, and upper vagina. Ovarian tissue is composed of embryonic yolk sac cells that give rise to the ova or germ cells, stromal cells that produce the steroid hormones, and mesothelium that provides the epithelial covering for the follicle cysts. These cell types give rise to germ cell tumors, sex cord–stromal tumors, and the epithelial tumors of the ovary, respectively. The traditional view of ovarian cancer pathogenesis is that all tumor subtypes arise from the OSE. In the “incessant ovulation hypothesis,” ovarian cancer develops from an aberrant repair process as a result of repeated rupture of the surface epithelium during each ovulatory cycle, thought to produce inflammation and scarring that serves as a nidus for carcinogenesis.9–11 A second proposed mechanism is related to hormonal and reproductive factors such as persistent exposure to gonadotropins and elevated estradiol levels that stimulate malignant transformation.12,13 Strong evidence exists that many borderline tumors and low-grade carcinomas of the ovary arise from cortical inclusion cysts (CICs) within the ovarian parenchyma. These benign cysts are composed of Müllerian epithelium that closely resembles the fallopian tube. CICs are thought to result from invaginations of the OSE into the ovarian stroma through repeated ovulation and aging, acquiring a Müllerian phenotype through metaplasia.8 An alternate explanation is that remnants of Müllerian-derived epithelia from the fallopian tube may adhere to the ovarian surface and become incorporated into CICs in a process known as endosalpingiosis. Nonetheless, as a result of hormone exposure, damage repair processes, and inflammation within the ovary, neoplastic transformation gives rise to a variety of Müllerian-cell type differentiations, including serous carcinomas that resemble the fallopian tube, mucinous tumors as seen in the endocervix, endometrioid tumors from the endometrium, and glycogen-rich clear cell cancers similar to secretory-phase endometrial glands.
High-grade serous ovarian carcinomas are infrequently associated with benign or borderline precursor lesions within the ovary, a contradiction of the OSE-CIC model. Furthermore, high-grade serous carcinomas of the ovary share distinct morphologic and genetic characteristics with high-grade serous carcinomas of other extrauterine sites, including serous fallopian tube carcinoma and primary peritoneal serous carcinoma. In this context, a second model of ovarian carcinogenesis has emerged with the recognition of the distal fallopian tube as the primary site of origin for many high-grade serous carcinomas. With rigorous pathologic processing, occult fallopian tube cancer has been detected in the fimbria of prophylactic salpingo-oophorectomy specimens from high-risk patients.14,15 Furthermore, TICs have been found in a large proportion of high-grade serous carcinomas initially designated as primary ovarian cancers.16 An adenoma-carcinoma sequence has since been described that involves a dysplastic tubal precursor lesion with loss of p53 detectable by immunohistochemical staining (“p53 signature”) with progression to a TIC characterized by increased proliferation, followed by the development of frank invasive serous carcinoma.7,17 The concept of the fallopian tube as the primary site of high-grade serous carcinoma suggests that previously undetected serous TICs may spread to the adjacent ovary or peritoneal cavity early in the disease process of serous carcinogenesis.
FIGURE 71.2. Ovarian cancer incidence and deaths in the United States from 2005 to 2012. (Courtesy of the American Cancer Society.)
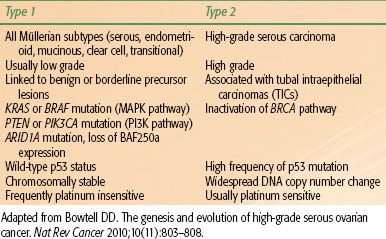
TABLE 71.1 TWO-PATHWAY MODEL OF EPITHELIAL OVARIAN CARCINOGENESIS
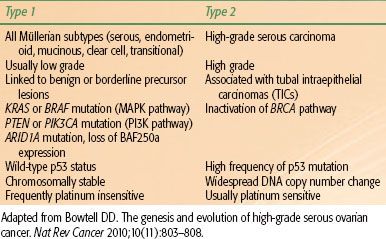
In the two-pathway model of ovarian carcinogenesis, type I tumors include all major histologic subtypes (serous, endometrioid, mucinous, clear cell, and transitional) with low nuclear and architectural grade that may be linked to well-defined benign precursor lesions, and type II tumors account for the bulk of high-grade serous carcinomas (Table 71.1). The type I tumors are associated with distinct molecular alterations, such as mutations in KRAS, BRAF, and PTEN, which are rarely found in type II serous tumors.18 Mutually exclusive KRAS and BRAF mutations, both of which activate the oncogenic MAPK signaling pathway, are observed in 65% of serous borderline tumors but are rarely seen in high-grade serous carcinomas.19 KRAS mutations also occur in Müllerian histologic subtypes, including 60% of mucinous, 5% to 16% of clear cell, and 4% to 5% of endometrioid carcinomas.18 Mutations in PTEN, which lead to constitutive activation of the related PI3 kinase signaling pathway, have been detected in 20% of endometrioid carcinomas,20 whereas activating mutations in PIK3CA have been identified in 30% of clear cell cancers.21 Somatic mutations in ARID1A, a novel tumor suppressor gene, were recently identified in 46% of ovarian clear cell cancers and 30% of endometrioid cancers. ARID1A mutation and corresponding loss of BAF250a expression, a key component in chromatin remodeling, were also seen in preneoplastic lesions and may represent an early event in the transformation of endometriosis.22 In contrast, type II tumors exhibit a high frequency of p53 mutation and widespread DNA copy number change. Inactivation of the BRCA pathway, a critical component of DNA repair by homologous recombination, is also a hallmark of high-grade serous carcinomas.23 Disruption of the BRCA pathway by germline or somatic mutation, epigenetic silencing, or microRNA regulation has been observed in >50% of serous cancers,24 and functional assays show defective formation of homologous recombination repair foci following DNA damage.25
Angiogenesis plays an important role in normal ovarian function, as heavy vascularization occurs at the beginning of each ovulatory cycle with predictable variation in the serum levels of vascular endothelial growth factor (VEGF). Epithelial ovarian cancers frequently overexpress VEGF, fibroblast growth factor, platelet-derived growth factor (PDGF), and angiopoietin.26 In preclinical models, expression of VEGF provides a survival advantage to transformed cells of the ovary. Other studies have found an association between preoperative serum VEGF level and clinical outcome.27 Targeting the tumor microenvironment in ovarian cancer is an attractive treatment strategy given the inherent genomic instability of high-grade serous cancers.
TABLE 71.2 FACTORS INFLUENCING THE RISK OF OVARIAN CANCER
 RISK FACTORS
RISK FACTORS
Epidemiologic studies have identified hormonal, genetic, and environmental factors that may play an important role in ovarian carcinogenesis (Table 71.2). The OSE-CIC model is consistent with well-established epidemiologic data indicating that suppression of ovulation results in substantial reduction of ovarian cancer risk. New insights into the pathogenesis of high-grade serous carcinomas have emerged through the study of high-risk populations with a genetic predisposition for the development of ovarian cancer. Nevertheless, the pathogenic mechanisms are not well understood and likely multifactorial, associated with both patient-related and environmental factors.
Patient-Related Factors
Risk factors associated with a higher frequency of ovulatory events, such as advancing age, low parity, infertility, early menarche, and late menopause, support the proposed mechanism of incessant ovulation.28–31 Although infertility is associated with ovarian cancer, the use of fertility drugs has not been conclusively linked.32–34 Suppression of ovulatory events from pregnancy, breast-feeding, and oral contraceptive use may explain the observed protective benefit.30,31,35 With oral contraceptive use for 4, 8, or 12 years, the risk of ovarian cancer is reduced by 40%, 53%, and 60%, respectively.36 In a collaborative meta-analysis of 45 epidemiologic studies from 21 countries, the use of oral contraceptives was significantly associated with a decreased risk of ovarian cancer in ever users (hazard ratio [HR] 0.73, p <.0001), with larger reductions observed for longer duration of use.37 The authors conclude that 200,000 ovarian cancers and 100,000 deaths have been prevented since the introduction of oral contraceptives 50 years ago.
Other patient-related risk factors such as polycystic ovarian disease and endometriosis may act through hormonal mechanisms attributable to elevated gonadotropins or by chronic inflammation. In a meta-analysis of eight case-control studies, women with polycystic ovarian disease had a 2.5-fold increase in ovarian cancer risk.38 Endometriosis has been shown to be an independent risk factor for ovarian cancer with an estimated rate of malignant transformation of 2.5%.39 Ovarian cancers that arise from endometriosis are most often low-grade tumors with endometrioid or clear cell histology and are associated with a better prognosis.40 Altered hormonal levels such as elevated androgens may also increase risk, whereas progestins have been shown to be protective.41 Large prospective cohort studies have demonstrated an increase in ovarian cancer risk and mortality with the use of exogenous estrogen and hormone replacement therapy.42–44 Surgical procedures, including hysterectomy and tubal ligation, are independently associated with a 34% reduction each in ovarian cancer risk, although the protective mechanism is unknown.29,45–47 Chronic inflammatory conditions such as pelvic inflammatory disease and tuberculous salpingitis were once believed to be causative factors in the development of fallopian tube malignancies, although this theory remains unproven.
Genetic Factors
Heredity tumors account for 10% to 15% of all ovarian cancers, and family history is the strongest risk factor after increasing age.48,49 The risk of developing ovarian cancer in the general population is approximately 1.4%, whereas the lifetime risk for a woman with one first-degree family member with ovarian cancer is 5% and climbs to 7% with two first-degree relatives. If a hereditary syndrome is present, the lifetime risk is on the order of 25% to 50% with an age of diagnosis approximately 10 years younger than women with sporadic disease.50
Three distinct familial ovarian cancer syndromes have been identified by pedigree analysis with autosomal dominant patterns of inheritance.51,52 BRCA1 and BRCA2 mutations are the most common genetic alterations, with up to 90% of all hereditary cases associated with a deleterious mutation of the BRCA1 gene, located on chromosome 17q21, or the BRCA2 gene, located on chromosome 13q22. The lifetime risk of ovarian cancer is approximately 40% in BRCA1 mutation carriers and 18% in BRCA2 mutation carriers.53 BRCA-associated ovarian cancers are most frequently invasive serous adenocarcinomas and less likely borderline or mucinous tumors.54 Mutation carriers are also more likely to present with advanced stage disease and have poorly differentiated tumors.55 Nonetheless, BRCA1 or BRCA2 mutation carriers have a more favorable clinical course with a significantly longer recurrence-free and overall survival compared to noncarriers. The improved outcome appears related to a higher sensitivity to platinum chemotherapy across first and subsequent lines of treatment.56,57,58–59,60
The importance of the fallopian tube in the pathogenesis of extrauterine serous carcinoma was initially recognized in BRCA1 and BRCA2 mutation carriers. In a prospective study of 483 BRCA1 mutation carriers, the incidence of fallopian tube cancer was reported as 120 times that of the general population.61 High-grade serous primary peritoneal carcinomas are also frequently observed in mutation carriers.62 Further attention was focused on the fallopian tube as the primary site of origin following reports of epithelial dysplasia and occult serous carcinomas in prophylactic salpingo-oophorectomy specimens from mutation carriers.63–66 In contrast to the standard pathologic sampling of the ampullary region of the fallopian tube in ovarian cancer cases, more extensive complete sectioning of the tube revealed an abundance of lesions in the fimbria of the distal tube.14,15–16 The detection of early serous carcinomas, or TICs, lent further support to the emerging concept of the fallopian tube as the primary site for extrauterine serous carcinoma. In this context, the National Comprehensive Cancer Network (NCCN) guidelines recommend that any woman with a diagnosis of ovarian, fallopian tube, or primary peritoneal carcinoma be referred to a cancer genetics professional for consideration of BRCA mutation testing.67 BRCA screening in a population of women with non-mucinous ovarian cancer detected germline mutations in 14% of women, including 22% with high-grade serous cancer, reinforcing the importance of offering testing to all patients.68
Hereditary nonpolyposis colorectal syndrome, or Lynch type II cancer syndrome, is responsible for the remaining 10% of hereditary ovarian cancers.69 In this syndrome, germline mutations of DNA mismatch repair genes lead to an increased risk of colorectal, stomach, endometrial, and ovarian cancer owing to underlying microsatellite instability. A total of seven mismatch repair genes have been identified with mutations in MLH1 and MSH2 accounting for 90% of the observed mutations in Lynch syndrome families.70,71 Mutations in MSH6 and PMS2 have been reported in the remaining 10% of families, whereas alterations in the remaining three identified genes are uncommonly observed. Inactivating mutations in specific genes may modify the underlying cancer predisposition. Women with MSH6 mutations are twice as likely to develop endometrial cancer compared to carriers of MSH2 or MLH1 gene mutations, which are the most common alterations underlying colorectal cancer risk.72 Women with MSH2 gene mutations have been shown to have a risk of epithelial ovarian cancer twice that of MLH1 carriers. In contrast to BRCA1 and BRCA2 mutation carriers, women with Lynch syndrome may present with a variety of nonserous epithelial tumor types, including endometrioid and clear cell histologies. Ovarian cancer associated with Lynch syndrome is more often diagnosed at an earlier stage with well to moderately differentiated tumor grade. The lifetime risk of ovarian cancer in women with Lynch syndrome is estimated at 3% to 14% and is most commonly diagnosed in the fifth decade of life.73 In women with a family or personal history suggestive of Lynch syndrome, tumor testing may be performed by microsatellite instability analysis or immunohistochemistical staining for mismatch repair genes. Direct sequencing of the mismatch repair genes is used for detection of germline mutations. Other less common germline mutations have been identified by genomic sequencing and involve the following genes: BARD1, BRIP1, CHEK2, MRE11A, NBN, PALB2, RAD50, RAD51C, and TP53.74
Other genetic disorders linked with nonepithelial ovarian cancers include Peutz-Jeghers syndrome, which is associated with an increased risk of sex cord–stromal tumors, and gonadal dysgenesis, associated with dysgerminomas and gonadoblastomas.
Environmental Factors
Environmental or physical causes of ovarian cancer have been investigated through numerous case-control studies. Women in developing countries have a lower incidence of ovarian cancer than those living in industrialized nations,75 although to date, no specific chemical carcinogens have been identified. Chronic exposure to asbestos-related products, including talc products, have long been implicated in the development of ovarian cancer.76 The Nurses’ Health Study reported a modestly elevated risk of invasive serous cancers with talc use (relative risk [RR] 1.4), although no association was detected with overall ovarian cancer risk.77 The development of mucinous ovarian cancer has been found to be associated with cigarette smoking (RR 2 to 2.2) but not other epithelial subtypes.78,79
Dietary and metabolic factors have also been explored in large population-based studies as possible contributors to ovarian cancer risk. To date, there have been no consistent associations of coffee or alcohol consumption with increased risk. Large epidemiologic studies also showed no relationship between consumption of animal fat and the development of ovarian cancer.80,81 In a recent Swedish population-based cohort study of the effect of body mass index on cancer risk in >35,000 women, a 36% higher risk of cancer was observed in obese women (body mass index ≥30) relative to women with body mass index in the normal range (18.5 to 25); cancer sites most strongly related to obesity were endometrium, ovary (risk for top quartile 2.09; 95% confidence interval [CI], 1.13 to 4.13), and colon.82 Obesity has also been associated with increased ovarian cancer mortality in a large prospective study of adults in the U.S.83 There is no clear relationship between exercise and ovarian cancer risk.
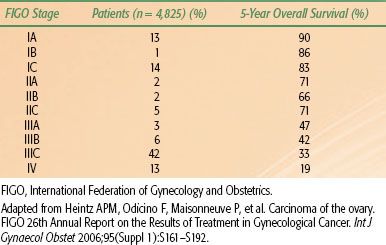
TABLE 71.3 EPITHELIAL OVARIAN CANCER STAGE DISTRIBUTION AND SURVIVAL BY STAGE
 SCREENING
SCREENING
In the absence of a reliable screening test, most women with ovarian cancer are diagnosed with advanced-stage disease. In contrast to women with localized disease (stage I/II) who have estimated 5-year survival rates of 70% to 90%, overall survival for women with advanced disease (stage III/IV) is poor, ranging from 20% to 45% (Table 71.3). The low sensitivity and specificity of the available screening modalities and the low prevalence of the disease in the general population have hindered the development of a robust screening test. Recent screening approaches have been associated with a low positive predictive value and unacceptable false-positive rates without impacting disease mortality in the general population or high-risk groups.
Several screening strategies have been investigated, including pelvic exam, the serum tumor marker CA-125, and transvaginal ultrasound (TVUS), either alone or in combination.84 CA-125 values >35 U/mL are observed in 80% of patients with epithelial ovarian cancer, including 90% of women with advanced-stage disease, but only in 50% of early-stage patients.85 CA-125 is nonspecific for ovarian cancer, as it can be elevated in benign gynecologic and nongynecologic conditions as well as nongynecologic cancers. Screening with CA-125 alone has been found to lack specificity in an average-risk population of postmenopausal women. Ultrasound alone is able to detect early-stage disease in an average-risk population,86–88 although the significant false-positive rate remains a concern. In a United Kingdom multimodality screening study, diagnostic surgeries were performed nine times more frequently to detect one cancer in the group screened by TVUS alone compared to multimodality screening (CA-125 and TVUS).89
Several large screening trials currently in progress in the United States, United Kingdom, and Japan have been designed to assess reduction in mortality with early detection of ovarian cancer. The Prostate, Lung, Colorectal, and Ovarian Cancer (PLCO) screening trial in the United States randomized 78,216 women aged 55 to 74 to annual screening with CA-125 and TVUS versus usual medical care. Following baseline screening, 570 surgical procedures, including 325 laparotomies, were performed; 29 tumors were detected, 9 of which were of LMP.90 The positive predictive value for the detection of invasive ovarian cancer was 3.7% for an abnormal CA-125, 1.0% for an abnormal TVUS, and 23.5% for both. Publication of the mortality data with 12-year follow-up showed no difference in the stage of cancer detected by screening (90% stage III or IV) and no reduction in cause-specific or overall mortality for women who underwent screening.91 Furthermore, diagnostic evaluation for women with false-positive screening resulted in a serious complication rate of 15%.
A second trial, the United Kingdom Collaborative Trial of Ovarian Cancer Screening (UKCTOCS), has accrued >200,000 postmenopausal women to evaluate multimodality screening with TVUS and serum CA-125. In the prevalence screen, the sensitivity, specificity, and positive predictive values of multimodality screening for invasive epithelial ovarian and tubal cancers were 89.5%, 99.8%, and 35.1%, respectively.89 The initial results of baseline screening were more promising than the U.S. study, with 43% of invasive cancers detected as stage I or II, which may reflect differences in study design and the diagnostic algorithm. The effect of multimodality screening on mortality is awaiting further follow-up. The multicenter, randomized Japanese study utilizes a similar screening strategy with TVUS and CA-125 and has accrued >80,000 women. Unlike the U.S. and UK trials, there was a nonsignificant trend for the detection of more stage I cancers in the screened population (63% vs. 38%), although mortality rates have not yet been reported.92
The present data do not support routine screening for ovarian cancer in the general population. All of the North American expert groups, including the U.S. Preventive Services Task Force, the American College of Obstetricians and Gynecologists (ACOG), the Society of Gynecologic Oncologists (SGO), and the Canadian Task Force on the Periodic Health Examination, recommend against routine screening in asymptomatic women. The incidence of ovarian cancer is relatively low in the general population; thus, the concerns for false-positive screening and the associated risks of exploratory surgery are significant.93 However, women at high risk for ovarian cancer with BRCA1 or BRCA2 mutations or hereditary nonpolyposis colorectal syndrome could potentially benefit from chemoprevention; screening with pelvic examinations, TVUS, and CA-125 on a biannual or annual basis; or prophylactic surgery.94 Although the efficacy of screening has been disappointing in the high-risk population,95 the ACOG, SGO, and NCCN guidelines recommend routine screening with pelvic exam, CA-125 and TVUS for women with hereditary ovarian cancer syndromes beginning at the age of 30 to 35, or 5 to 10 years earlier than the age of diagnosis of ovarian cancer in the family. The U.S. National Institutes of Health Consensus Development Panel recommends that prophylactic salpingo-oophorectomy be considered in women with ovarian cancer syndromes at age 35 years or after childbearing is complete, as the risk reduction with salpingo-oophorectomy is 80%.96,97 Removal of the ovaries also reduces the risk of breast cancer in BRCA mutation carriers.98
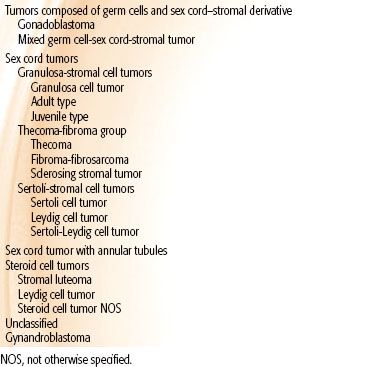
TABLE 71.4 WORLD HEALTH ORGANIZATION CLASSIFICATION OF OVARIAN TUMORS

 HISTOLOGIC CLASSIFICATION
HISTOLOGIC CLASSIFICATION
The World Health Organization and International Federation of Gynecology and Obstetrics (FIGO) have adopted a unified classification of the common epithelial, germ cell, sex cord, and stromal tumors99 (Table 71.4). Most ovarian malignancies (60% to 65%) are epithelial, with germ cell tumors (20%), sex cord–stromal tumors (5%), and metastases to the ovary (5% to 10%) accounting for the remainder.100 Serous tumors are most common, comprising 50% to 60% of epithelial tumors. Other subtypes include mucinous carcinoma in 10%; endometrioid carcinoma, 8%; clear cell carcinoma, 3% to 5%; transitional, 3% to 5%; and undifferentiated carcinoma, 1%. Bilateral presentation occurs frequently in epithelial tumors, most commonly in serous tumors followed by endometrioid (15%) and mucinous tumors (5% to 10%).
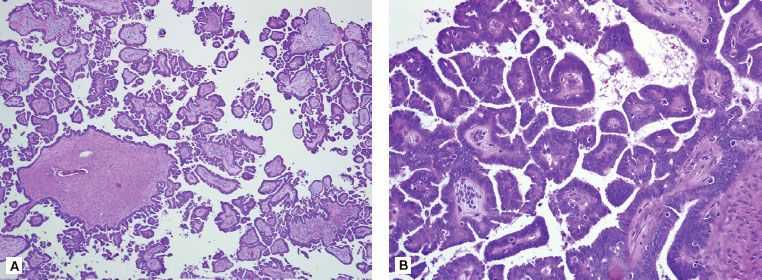
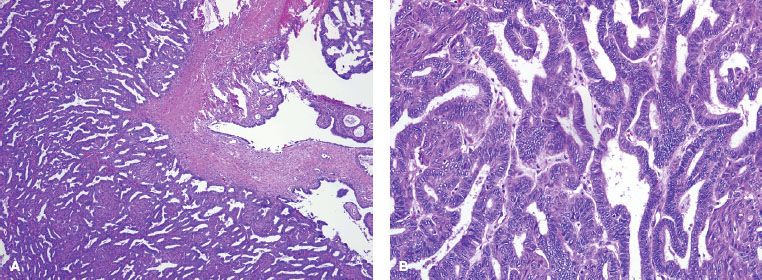
FIGURE 71.3. Serous tumor of low malignant potential of the ovary at low power (A) and high power (B). At low power, note the progressively branching papillary fronds with fibrovascular support and detached papillary clusters; at high power, note the serous epithelium with nuclear hyperchromasia and cytologic atypia. By definition, there is no destructive stromal invasion.
High-grade serous carcinomas are often widely disseminated at diagnosis and account for most deaths from ovarian, tubal, and peritoneal cancers. In contrast, 5-year survival for low-grade serous carcinoma is 85%. Most serous tumors present as large ovarian masses and grossly appear nodular with multiple papillary projections and cysts filled with clear serous fluid. A two-tiered grading system separates high-grade serous carcinomas from low-grade tumors,101,102 which have a similar prolonged natural history to noninvasive borderline tumors, or tumors of LMP.103 As discussed previously, there is increasing evidence that high grade serous carcinomas originate in the fimbria of the fallopian tube and may spread rapidly to the adjacent ovary and peritoneal sites. Low-grade tumors appear to follow a stepwise progression from borderline tumor to invasive carcinoma and involve molecular pathways distinct from their high-grade counterparts.104–106 Although treatment is similar for all serous tumors, low-grade tumors have been shown to be less responsive to chemotherapy.103,107,108
LMP or borderline tumors have nuclear abnormalities and mitotic activity intermediate between benign and malignant tumors of similar cell type but lack stromal invasion (Fig. 71.3). Borderline tumors are a subcategory of ovarian malignancies that account for 10% to 20% of all epithelial neoplasms.109,110 The prognosis, surgical approach, and postoperative treatment recommendations are vastly different as compared with those of their invasive counterparts. The majority of LMPs (75%) present with stage I disease, which directly contrasts with the 75% advanced stages in the invasive epithelial tumors. The 5- and 10-year survival rates for women with LMP tumors are >95%. Significant heterogeneity exists in the biologic behavior of borderline tumors. Women with nonlocalized LMP tumors of the ovary have decreased survival compared to those with localized LMP tumors but are similar to that of women with localized, well-differentiated epithelial ovarian carcinoma.111 Other pathologic features that affect prognosis include the cell type, tumor stage, implant type (invasive vs. noninvasive), the presence of micropapillary architecture, and microinvasion. Extensive sampling of all pathologic specimens is required to firmly establish the diagnosis.
Most endometrioid and clear cell carcinomas arise in foci of endometriosis and follow an adenoma to borderline tumor to carcinoma sequence. Endometrioid carcinoma of the ovary resembles its endometrial counterpart (Fig. 71.4), and synchronous primary cancers are detected in 10% of women with ovarian cancer and 5% of women with endometrial cancer.112 Bilateral ovarian involvement, small multinodular ovaries, and surface and hilar spread suggest metastatic involvement from an endometrial primary, particularly if the endometrial tumor is high-grade, deeply invasive, and associated with lymphovascular invasion. A large, cystic, unilateral tumor of low grade arising in a focus of endometriosis likely represents a primary ovarian tumor. Endometrioid tumors appear to have a better prognosis than serous cancers regardless of tumor stage.113 Multiple synchronous primary tumors may represent a field defect related to endometriosis, which is associated with improved survival.114
Mucinous tumors follow a similar progression from cystadenoma to borderline tumor prior to invasion. All subtypes are more likely to be diagnosed when confined to the ovary. Mucinous cancers may grow to a very large size, often measuring up to 20 cm in diameter and filled with large pockets of thick, necrotic, mucinous debris.115,116 Ovarian mucinous tumors closely resemble mucin-secreting tumors originating from other sites—most commonly the gastrointestinal tract. The pathologic distinction between primary and metastatic mucinous carcinoma may be challenging, despite the use of immunohistochemical analysis. Further clinical evaluation is often required to exclude a clinically occult nonovarian primary source. Secondary involvement of the ovary may also occur in association with pseudomyxoma peritonei arising from a low-grade tumor often of appendiceal origin.117
Clear cell carcinoma is characterized by clear and hobnail cells with a similar histologic appearance to clear cell carcinoma of the kidney, endometrium, and vagina. Ovarian clear cell carcinoma is often seen in association with venous thromboembolism and hypercalcemia. Although more likely to be stage I than serous carcinoma, clear cell histology is associated with a lower response rate to platinum-based chemotherapy, higher recurrence rate, and worse survival.118 The transitional cell tumors, including Brenner tumors, are benign in most cases (98%) and carry a favorable prognosis.
Ovarian germ cell tumors comprise <5% of ovarian malignancies and are classified by the World Health Organization (Table 71.4). Dysgerminomas are the most common of the germ cell tumors and occur bilaterally in 10% to 20% of cases. The other germ cell tumor types are typically unilateral. Endodermal sinus tumors, also known as yolk sac tumors, are characterized by Schiller-Duvall bodies. Embryonal carcinomas are rare and tend to occur in younger populations; they may be seen with nongestational choriocarcinomas as part of mixed germ cell tumors (10% of all germ cell tumors). Immature teratomas are characterized by immature elements from the germ layers. The grade, treatment recommendations, and outcome are directed by the amount of immature neural elements.
Sex cord–stromal tumors are also classified by the World Health Organization (Table 71.3), with granulosa cell tumors being the most common (70%). Histologically, the granulosa cell tumors are composed of granulosa cells that have a pale, grooved, “coffee bean” nuclei or a rosette of cells surrounding eosinophilic material, a Call-Exner body. Thecomas (hormonally active) and fibromas (hormonally inactive) are both clinically benign tumors and are most common in middle-age women.
The most common histopathology of primary fallopian tube malignancies is papillary serous adenocarcinoma. Other less common Müllerian subtypes include endometrioid, clear cell, and malignant mixed Müllerian tumors.119,120 Rare reports of squamous cell carcinoma, immature teratoma, glassy cell tumor, and sarcoma have also been described. Benign tumors are found even less frequently than malignant neoplasms. Metastatic involvement of the fallopian tube is reported in up to 12% of women with uterine cancer and 4% with cervical cancer.121,122
FIGURE 71.4. Endometrioid adenocarcinoma of the ovary. A: At low power, note the tumor composed of back-to-back endometrioid glands. B: At high power, note the squamous metaplasia, commonly seen in this epithelial variant. Assignment of tumor grade is based on similar criteria as for carcinomas arising in the endometrium. This tumor would qualify as well differentiated or grade 1.
 PATTERNS OF SPREAD
PATTERNS OF SPREAD
The primary mode of spread for epithelial ovarian and fallopian tube cancers is transperitoneal, as malignant cells exfoliate into the peritoneal cavity. Intraperitoneal spread is favored by intestinal peristalsis and negative hydrostatic pressure below the diaphragm. The exfoliated tumor cells follow the intra-abdominal fluid stream passing along the paracolic gutters toward the diaphragm, predominately on the right side, before implantation on any peritoneal surface.123,124 Metastatic deposits are frequently seen in the posterior cul-de-sac, paracolic gutters, diaphragmatic surfaces, liver capsule, intestinal surfaces, and omentum. Metastases may also be found in the uterus or contralateral ovary from peritoneal spread or flow through the fallopian tubes. Dense tumor “caking” can cause infiltration into the abdominal organs creating a mass effect on the omentum, ureter, bowel, liver, pancreas, spleen, or adrenals, resulting in advanced disease stage at presentation with associated ascites.
Lymphatic drainage constitutes the second most common pattern of spread. The lymphatic capillaries of the ovary converge on the hilus and follow the ovarian blood vessels in the infundibular ligament to drain to the para-aortic nodes at the level of the renal hilum. Lymphatics also drain along the broad ligament to the hypogastric and external iliac nodes in the pelvis. Less frequently, spread can occur to the inguinal nodes via the round ligament.125 Approximately 10% to 15% of women with disease that appears confined to the ovary have para-aortic lymph node involvement,126 which becomes increasingly common in advanced-stage disease. Because of the rich lymphatic supply of the fallopian tubes, lymph node involvement is common even in the absence of tubal musculature involvement.127 Pelvic and para-aortic lymph node involvement has been reported in 10% to 30% of women with fallopian tube cancer at diagnosis.128
Transdiaphragmatic spread occurs to the pleural cavity and is the most common finding in stage IV disease. Hematogenous spread is infrequent at the time of presentation, with only 2% to 3% of patients with parenchymal liver or lung disease. Brain metastasis is also rare. However, >50% of recurrences occur both within and outside the peritoneal cavity at the time of treatment failure.
 CLINICAL PRESENTATION
CLINICAL PRESENTATION
As ovarian cancer has insidious growth and is asymptomatic in early-stage disease, most women do not present until symptoms arise from advanced disease progression. Vague gastrointestinal complaints of dyspepsia, nausea, early satiety, bloating, constipation, or obstipation are common presenting symptoms, as are genitourinary symptoms including frequency, urgency, or incontinence. Other ill-defined symptoms include fatigue, back pain, pain with intercourse, and menstrual irregularities. These nonspecific symptoms can be present for several months but may not trigger diagnostic evaluation until after the symptoms fail to clear with other medical therapy.129 Detection of early-stage disease may occur by palpation of an asymptomatic adnexal mass on routine examination, although most adnexal masses require moderate size for palpation. In premenopausal women, most of these masses are benign, as ovarian cancer represents <5% of adnexal neoplasms. An adnexal mass in a postmenopausal woman has a higher likelihood of malignancy, and surgical exploration is often indicated. Physical examination findings such as a fixed pelvic mass, palpable upper abdominal mass, and ascites are highly suggestive of an ovarian malignancy. According to a consensus statement on the symptoms of ovarian cancer published by the Gynecologic Cancer Foundation, SGO, and American Cancer Society, new and persistent symptoms of bloating, pelvic or abdominal pain, difficulty eating, early satiety, or urinary urgency or frequency should prompt women to seek medical evaluation.
A portion of women with fallopian tube carcinoma may present with early clinical signs and symptoms. Two triads have been described as pathognomonic for fallopian tube malignancy: (a) pelvic pain, pelvic mass, and leukorrhea and (b) vaginal bleeding, vaginal discharge, and lower abdominal pain. However, the percentage of patients presenting with either triad of symptoms has been reported as low as 11%.130 Another classic sign—hydrops tubae profluens—is a sudden emptying of accumulated fluid in the distended fallopian tube that causes profuse, watery, serosanguineous vaginal discharge. The discharge is often accompanied by a decrease in pelvic mass size on physical examination. In a meta-analysis of 122 patients with primary fallopian tube cancer, hydrops tubae profluens was a presenting sign in only 9%.121 In other series,124,127 it has been specifically reported that neither a triad nor hydrops tubae profluens was present in any of the patients reviewed. When clinically apparent, the fallopian tube may be dilated and mimic more benign pathologic processes such as hydrosalpinx, pyosalpinx, or hematosalpinx.131 In more advanced disease with tubal wall invasion, extension of a necrotic mass to involve the ovary may give the initial impression of a tubo-ovarian abscess.
Germ cell and stromal cell malignancies present at an earlier stage than epithelial ovarian or fallopian tube cancers, often related to abdominal discomfort or symptoms of excessive estrogen or androgen production. Granulosa cell tumors may lead to precocious puberty in young girls, and Sertoli-Leydig cell tumors may cause virilization.
 DIAGNOSTIC WORKUP
DIAGNOSTIC WORKUP
Evaluation of a pelvic mass will be influenced by the patient’s age, clinical presentation, and imaging features. An ovarian mass is more likely to be a malignant neoplasm in the pediatric, perimenopausal, and postmenopausal age groups and benign during the reproductive years. Ultrasound is often the first, noninvasive step for the evaluation of a pelvic mass. Sonographic characteristics suggestive of malignancy include irregular borders; a solid component that is not hyperechoic and often nodular or papillary; Doppler demonstration of flow in the solid component; dense multiple irregular septa (>2 to 3 mm); and the presence of ascites, peritoneal masses, enlarged nodes, or matted bowel. Computed tomography (CT) and magnetic resonance imaging (MRI) may be useful preoperatively for surgical planning to determine the extent of intra- and extra-abdominal disease. Although limited as a screening tool, an elevated CA-125 level may suggest more advanced or greater bulk of disease and high-grade serous histology but in general is a weak predictor of surgical resection.132,133 Human chorionic gonadotropin (β-hCG), α-fetoprotein (AFP), total inhibin, and lactate dehydrogenase levels may aid in the diagnosis of and treatment for nonepithelial germ cell ovarian tumors.
Other conditions may mimic ovarian cancer in their presentation, including colon, gastric, and appendiceal carcinomas as well as metastatic breast cancer and primary lymphoma. Although ideally the primary site of disease is determined in the preoperative setting, the diagnosis often cannot be made accurately until the time of surgery, and frozen section pathologic evaluation may guide surgical approach. Primary malignancies of the fallopian tube have been difficult to diagnose prior to surgical exploration, as the clinical presentation may be similar to that of salpingitis, ovarian abscess, pelvic inflammatory disease, or even ectopic pregnancy. Occult tubal primaries may be detected only by careful pathologic processing.
Referral to a gynecologic oncologist should be considered for any woman with a suspicious pelvic mass, family history of ovarian or fallopian tube cancer, or elevated CA-125.134 Initial evaluation should include a thorough history and full physical and pelvic examination, laboratory studies (complete blood count, chemistries, CA-125, carcinoembryonic antigen, CA 19–9), and imaging (abdominal CT/MRI, directed ultrasound, chest x-ray). Further evaluation with mammography, upper gastrointestinal endoscopy, or colonoscopy may be indicated in some scenarios. Comorbid conditions may prompt additional evaluations, including cardiac risk assessment, pulmonary function testing, and nutritional evaluation.
TABLE 71.5 FIGO STAGING FOR OVARIAN CARCINOMA

 SURGICAL STAGING
SURGICAL STAGING
Surgical advances in staging and the therapeutic benefit of a maximal safe tumor resection have improved the progression-free and overall survival of women with ovarian cancer over the past two decades. Traditional staging for ovarian cancer is based on the FIGO staging system shown in Table 71.5.135 A parallel American Joint Committee on Cancer system of TNM staging also exists, with strong correlations between stage and the prognostic value of these substages. Staging of primary fallopian tube cancer was established by FIGO in 1992 based on the ovarian cancer staging system. A proposed modification was later published to permit staging of noninvasive tubal carcinomas and fimbrial carcinomas as well as the inclusion of substaging based on depth of invasion119 (Table 71.6).
Comprehensive surgical staging is the mainstay of diagnosis and initial treatment for ovarian, fallopian tube, and peritoneal cancers.136 In apparent early-stage cancers, surgical evaluation is required for accurate pathologic staging to guide adjuvant treatment recommendations. In patients with advanced disease, surgery represents the initial treatment by providing optimal cytoreduction. Comprehensive surgical staging begins with an exploratory laparotomy via a vertical midline incision followed by collection of peritoneal washings or any ascitic fluid for cytology. The surgeon performs a thorough inspection of all visceral and peritoneal surfaces within the abdomen and pelvis, with particular attention to the intestinal serosal surfaces, mesentery, paracolic gutters, hemidiaphragms, gallbladder, and liver. Systematic biopsies of the bladder serosa, anterior and posterior cul de sac, paracolic gutters, hemidiaphragms, and any suspicious adhesions or implants should be obtained. Total hysterectomy, bilateral salpingo-oophorectomy, infracolic omentectomy, and pelvic and para-aortic lymph nodal sampling may be performed in addition to the intact removal of the adnexal mass when possible. Given the significant risk of contralateral involvement, bilateral lymph node assessment is frequently performed.137,138 In patients with advanced disease, bowel resection, diaphragm stripping, splenectomy, nodal dissection, and radical resection of peritoneal tumor nodules are often necessary to achieve the desired surgical outcome of maximal debulking. Recently, the role of routine appendectomy has been questioned and is not recommended in the absence of visible pathology.139
In select cases, preservation of the contralateral unaffected ovary and the uterus can be achieved in young women wishing to retain fertility who have stage I and/or low-risk tumors (early-stage, low-grade invasive tumors, LMP lesions, or malignant stromal or germ cell tumors).136 In the setting of unilateral salpingo-oophorectomy, comprehensive surgical staging should be performed to exclude occult disease. Exceptions to upfront surgical management include patients with significant medical comorbidities who are poor operative candidates and patients with a complex ovarian cyst where extraovarian disease has not been excluded. Neoadjuvant chemotherapy followed by interval cytoreduction may be considered in patients with bulky stage III or IV disease. Before initiation of neoadjuvant chemotherapy, the pathologic diagnosis should be confirmed by biopsy, fine-needle aspirate, or paracentesis. The NCCN guidelines recommend that patients who are evaluated for neoadjuvant chemotherapy be seen by a fellowship-trained gynecologic oncologist before being deemed a nonsurgical candidate.136
TABLE 71.6 MODIFIED FIGO FALLOPIAN TUBE STAGING