Kevin Hsueh, Jeffrey Bruce Greene
Outpatient Parenteral Antimicrobial Therapy
Outpatient parenteral antimicrobial therapy (OPAT) refers to the treatment of infections by intravenous therapy in settings other than acute-care hospitals or subacute health care facilities. The earliest published report of OPAT was in 1974 by Rucker and Harrison,1 who treated children with cystic fibrosis for exacerbation of lung infections. This came 40 years after the discovery of sulfonamides and 30 years after the parenteral antibiotic therapy era began with the availability of penicillin and chloramphenicol. Until that point, hospitals had been considered the preferred site for the management of complicated infections. Using parenteral antibiotics in settings other than a hospital is not simply a matter of choosing an alternative venue of care. The decision to treat and monitor a patient outside a hospital requires specific skill sets, along with a firm foundation in the management of infectious disease therapies.
A number of nearly concurrent developments served to propel the use of parenteral antibiotics in outpatient settings. The first was the development, in the late 1970s, of elastomeric venous access devices with complication rates low enough to serve as stable antibiotic delivery platforms.2 In the early 1980s, antimicrobials such as ceftriaxone, having pharmacokinetic, clinical efficacy, and safety profiles advantageous for outpatient administration, started to be widely used.3,4 Finally, in addition to the clinical advances, financial incentives developed that encouraged earlier discharge from the hospital. In 1983, diagnostic-related group (DRG) models for payment of clinical services were adopted nationally in the United States by the Health Care Financing Administration, now called the Centers for Medicare and Medicaid Services (CMMS). Other third-party payers subsequently adopted the DRG system, in which compensation to hospitals for inpatient care was no longer remitted on a fee-for-service manner but instead as a lump-sum payment based on a weighted estimate of cost for specific diagnoses multiplied by a fixed average length of stay. It was quickly understood that prolonged hospital stays represented financial liability, and means were sought to transfer patients from the acute-care setting earlier.5
There are many theoretical advantages to OPAT. Studies comparing parenteral antimicrobial treatment in the outpatient setting with hospital care have repeatedly shown significantly lower costs of OPAT across a wide range of infections. Although there is a reimbursement disparity for OPAT among different types of payers, daily costs are in the range of 25% of the daily costs of in-hospital treatment.6–8 Of importance, there appears to be an acceptably high rate of successfully completed courses of therapy compared with inpatient treatment.9–13 Furthermore, there is theoretically a lower risk of secondary nosocomial infections, such as Clostridium difficile colitis, methicillin-resistant Staphylococcus aureus (MRSA) or infection by multiple drug-resistant bacteria, which some studies have indicated.14–16 From the patient and family’s point of view, OPAT allows treatment of serious infections in familiar surroundings, with loved ones present, and in some cases, affords the ability to remain gainfully employed during the course of therapy. This is especially important for particularly sensitive populations, such as children and the elderly, where the unfamiliar and often frightening inpatient setting can actively hamper or obstruct therapy.17,18
Despite the continued growth of OPAT in the United States and around the world, there is still a paucity of well-designed studies comparing it with hospital-based antimicrobial therapy. There are few randomized controlled outcomes trials in the literature. The focus has instead been on cost and not efficacy. Even then, much of the recent cost savings data comes from cost-controlled health care systems, such as Canada and the United Kingdom. Therefore, these studies may not be representative of costs of OPAT in the less-regulated U.S. health care system. As OPAT became more commonly used in the United States, the diversity of infections and the case severity of patients treated increased in parallel. By the late 1990s, the organic growth of OPAT in the United States begged for a comprehensive database, similar to ones that those countries with national health services compile, so as to better analyze the risks, benefits, and outcomes. An attempt to address this issue in the United States came in the form of the OPAT Outcomes Registry (1997 to 2000), which studied more than 11,000 patients from 24 contributing medical centers, providing important clinical data.19 The registry reported critical information, such as the number and types of infections treated, the antimicrobial usage frequency, any incidence of antimicrobial side effects, and the percentage of treatment regimens completed versus those that were terminated early for toxicity or other reasons (Tables 53-1 and 53-2). Unfortunately, the registry could not be maintained for lack of resources, leaving an ever-widening gap in data collection that has yet to be filled.
Decision Making in Choosing Patients for OPAT
Despite decades of growth in OPAT use, the number of patients treated yearly in this manner still represents a small percentage of total patient-days of intravenous antibiotic use. This is because not every patient is suitable for OPAT, and not every physician has the skills or resources necessary for its use. To assess a patient for appropriateness for OPAT, it is necessary to consider a wide range of issues. This requires the input of a clinician who is versed in the principles of infectious diseases and who has experience in managing patients in the outpatient setting (Table 53-3).
TABLE 53-3
Decision Making in Outpatient Parenteral Antimicrobial Therapy
| Determine the clinical syndrome | Bacteremia/fungemia, bacterial endocarditis, soft tissue/joint/bone/hardware, pulmonary, genitourinary, Lyme disease,* HIV-associated opportunistic infections, intra-abdominal abscess, CNS infections |
| Consider the pathogen | Organism(s) identified, predictable pathogen(s), sensitivities identified, demonstrable response to therapy |
| Choose an antimicrobial | Good therapeutic index, drug levels predicted adequate for the infected site, expense/payer preference, ability or need to monitor drug levels |
| Weigh OPAT-limiting host factors | Advanced age, comorbid diseases (DM, hepatic/renal failure, neutropenia, cancer, immunodeficiency states), co-administered immunosuppressive therapies (corticosteroids, TNF inhibitors, chemotherapy), history of multiple antibiotic allergies, history of recurrent Clostridium difficile infection |
| Assess the outpatient treatment setting | Stable home, adequate clean treatment space, safe location for nursing visits, access to phone/Internet communication, patient/family able to self-treat or cooperate with caregivers |
| Recognize and manage payer restrictions, and assume a leadership role for patient’s care | Indispensable components for care need to be covered; antimicrobial provision, infusion supplies, intravenous catheter care, nursing, physician oversight. Concrete assignment of who is monitoring patient for therapeutic efficacy and side effects, and who is the contact person responsible for regimen changes is necessary |
* Use of intravenous antibiotic therapy for treatment of Lyme syndromes other than neuroborreliosis or carditis is controversial. Please refer to Chapter 243 for more specific recommendations regarding Lyme disease.
CNS, central nervous system; DM, diabetes mellitus; HIV, human immunodeficiency virus; OPAT, outpatient parenteral antimicrobial therapy; TNF, tumor necrosis factor.
Outpatient parenteral therapy may be considered when an infection requires treatment and no oral antimicrobial is appropriate, usually in the context of prolonged therapeutic courses. Ideally, most referrals for OPAT are for patients who have already begun their therapy in the hospital setting and have demonstrated a clear laboratory and clinical response to the planned therapeutic agent. Most patients receive the remainder of their antibiotic infusions at home after being taught how to self-administer, or, occasionally, entirely from a trained infusion nurse. In recent years, there has also been a modest growth in the numbers of patients referred for OPAT directly from physician clinics or emergency departments.
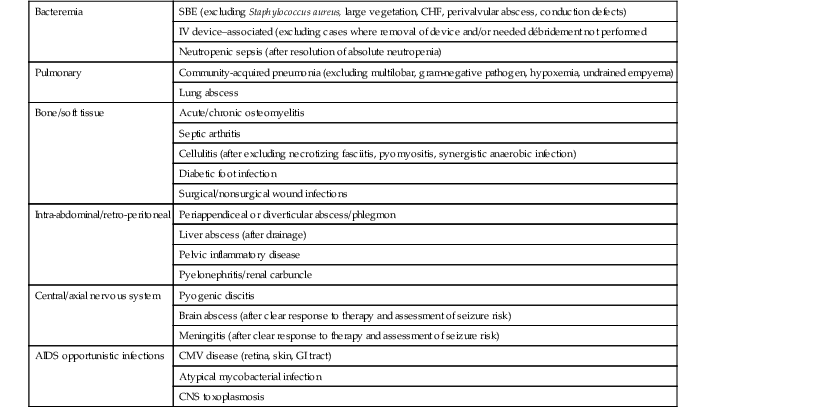
Making an accurate infectious disease diagnosis is the first key step in determining the suitability of OPAT. Conditions for which there is a clear standard of care, with available culture and sensitivity data, and for which there are easily obtainable clinical, laboratory, or radiologic parameters with which to assess the response to therapy are the preferred situations for application of OPAT (Table 53-4). For example, an appropriate referral to OPAT would be a patient with subacute bacterial endocarditis caused by a viridans strain of Streptococcus, which is without evidence of embolic complications, valvular dysfunction or large vegetations, and whose fever and bacteremia have resolved.20,21 However, not all cases successfully managed in the outpatient setting are so ideal. Frequently, OPAT is used in soft tissue or bone infections, where the identification of the causative pathogen(s) is not known, or in patients previously treated with antimicrobials, making isolation of a pathogen difficult.22 The literature is also rife with anecdotal case reports where OPAT has been used for virtually every conceivable kind of infection. However, there are clearly some infections for which OPAT should not be recommended. Patients with hemodynamic instability, hyperpyrexia, altered mental status, or poor performance status should never be discharged for home therapy.23,24 Similarly, patients on empirical or multiple antimicrobial agents for what would be predicted to be polymicrobial infections pose special challenges. Higher risks of drug toxicity or breakthrough bacteremia with resistant organisms should generally exclude such persons from outpatient treatment. The most prudent advice is to limit referrals for OPAT to those infections that do not pose imminent or likely threats to patient survival and have demonstrated a clear response to the class of antibiotics that will be used at home.
Infections that demand combined antimicrobial and surgical management should not be referred for OPAT until both modes of treatment are well underway. This might entail such interventions as percutaneous drainage of a liver abscess or empyema, stenting of an obstructed ureter, débridement of infected bone, or incision and drainage of a soft tissue abscess.
Identification of the causative organism(s) may influence the decision to consider outpatient therapy. In general, bacteremia demands an exhaustive evaluation to understand the source, pathogenesis, and potential effects before a patient is referred for OPAT. Polymicrobial bacteremia is especially worrisome. Patients infected with more pathogenic bacteria, such as MRSA, the Enterobacteriaceae, and clostridial species, are more likely to experience poor outcomes.25–28 Especially challenging are those patients in whom no causative organisms are recovered. An adequate period of observation on the intended home antibiotic therapy is essential before discharge from the hospital.
Host factors figure prominently in predicting successful antimicrobial therapy outcomes. Having patients of advanced age; those with comorbidities, such as diabetes mellitus, malignancy, chronic liver, or renal disease; those co-administered corticosteroids or other immunosuppressant agents; or those with neutropenia are but some of the factors that should argue against OPAT. In addition, patients with multiple medication allergies are more likely to experience OPAT complications, as are patients who have had recurrent Clostridium difficile infection.
The choice of antibiotic for OPAT is influenced by the isolated or suspected pathogen, the site of infection and the drug level achievable at those sites, pharmacokinetics of the drug, the patient’s renal and hepatic function, allergy history, and any anticipated toxicity of long-term therapy (Table 53-5).29 Although use of the narrowest-spectrum agent is usually advisable, in OPAT, selection of antimicrobials is often influenced by convenience of administration and the therapeutic margin of safety. For example, an infection caused by a methicillin-sensitive Staphylococcus aureus might be treated with ceftriaxone in the OPAT setting, rather than a narrower-spectrum drug such as oxacillin or nafcillin.30 Ceftriaxone has once-daily dosing (because of a longer half-life), is more stable at room temperature, and is less likely to cause phlebitis than the semisynthetic penicillins. Aminoglycosides may be used daily at higher doses (5 mg/kg), and extended intervals to provide improved bactericidal activity and postantibiotic effect with less toxicity.31 Knowledge of drug stability characteristics is crucial when reconstituted agents are stored for days in the home for patient self-administration. Drugs such as the penicillin derivatives might be administered by intermittent administration via a compact programmable pump worn by the patient.32,33 Unfortunately, despite their great clinical utility and safety enhancement, many payers will not authorize the use of these devices because of their added expense.
TABLE 53-5
Properties of Commonly Used OPAT Antimicrobials (Modified from 2004 IDSA Guidelines)
| DRUG | HALF-LIFE (hr) | STABILITY AT 5° C/25° C | PHLEBITIS RISK* | ISSUES OF CONCERN DURING OPAT |
| Caspofungin | >48 | 24 hr/24 hr | 1 | |
| Cefazolin | 1-2 | 10 days/1 day | 1 | |
| Ceftazidime | 1.4-2 | 21 days/2 days | 1 | |
| Ceftriaxone | 5.4-10.9 | 10 days/3 days | 1 | |
| Clindamycin | 2-3 | 32 days/16 days | 1 | |
| Daptomycin | 8.1 | 12 hr/48 hr | 1 | Occasional myopathy; CPK should be checked weekly. Can falsely prolong INR without changing anticoagulation; INR should be checked at least 24 hr after daptomycin dosing. |
| Ertapenem | 4 | 24 hr/6 hr | 2 | LFTs should be checked weekly. |
| Gentamicin | 2.3 | 30 days/30 days | 1 | Ototoxicity and nephrotoxicity common. Drug levels, kidney function must be checked twice weekly. Hearing and vestibular function must be monitored frequently. |
| Meropenem | 1.5 | 24 hr/4 hr | 1 | LFTs should be checked weekly. |
| Nafcillin | 0.5-1.5 | 3 days/1 day | 3 | Occasional hepatotoxicity; LFTs should be checked weekly. Higher risk of thrombophlebitis and DVTs. Frequent dosing requires advanced continuous infusion systems. |
| Oxacillin | 0.3-0.8 | 7 days/1 day | 2 | |
| Penicillin G | 0.4-0.9 | 14 days/2 days | 2 | |
| Tobramycin | 2-3 | 4 days/2 days | 1 | Ototoxicity and nephrotoxicity common. Drug levels, kidney function must be checked twice weekly. Hearing and vestibular function must be monitored frequently. |
| Vancomycin | 4-6 | 63 days/7 days | 2 | Occasional nephrotoxicity; kidney function should be checked weekly. Drug level monitoring recommended. Initial infusions must be monitored for red man syndrome. |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree