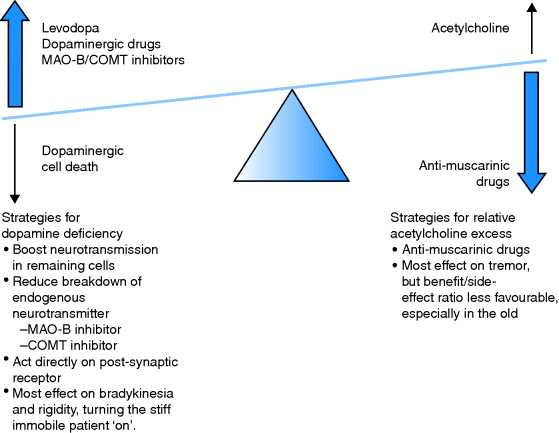
Age Changes and Clinical Examination
Symptomatic Classification of Neurological Disease in the Elderly
- Raised intracranial pressure, but fewer than 10% of patients with brain tumour present with headache alone.
- Pain radiating from cervical spondylosis.
- Giant-cell arteritis – tender temporal arteries, tenderness over proximal muscles, high ESR/CRP.
- Psychological – but the prevalence of ‘tension’ headaches declines with age, consider depression.
- Paget’s disease of the skull is occasionally painful when active – often obvious.
- Migraine, but new-onset is unusual over 50 years.
- Trigeminal neuralgia – mean age of onset is around 50 years; it rarely starts in old age.
- Dental problems.
- Sinusitis.
- Giant-cell arteritis (pain on chewing).
- Post-herpetic neuralgia (look for post-inflammatory pigmentary change in a trigeminal dermatome).
- Vascular disease.
- Space-occupying lesion.
- Unilateral PD.
- Cord compression – either vascular or space-occupying lesion.
- CSF infection.
- Guillain–Barré syndrome.
- Pressure from disc, bone or collection of pus.
- Neuropathy.
- Proximal myopathy.
- Cerebellar disease.
- Drug-induced.
- Cerebrovascular disease.
- Middle-ear disease.
- Myxoedema.
- PD.
- Drugs – especially phenothiazines.
- Disuse.
- Joint/bone problems.
- Spasticity of multi-infarct dementia.
- Nerve entrapment.
- Motor neurone disease (MND).
- Diabetes – mononeuritis.
- Meningitis, encephalitis or sepsis at any site.
- Raised intracranial pressure.
- Drugs (sedatives, hypnotics).
- Biochemical disturbances.
- Delirium.
- Lewy body dementia.
- Stroke (large lesions in the cerebral hemispheres, small lesions in the brain stem).
- Space-occupying lesion.
- Fits.
- Drugs (sedatives, hypnotics, alcohol).
- Poisoning (accidental – remember carbon monoxide, self-harm and iatrogenic).
- Biochemical disturbance (do not miss hypoglycaemia).
- PD.
- Drugs (anti-Parkinsonian treatment, neuroleptics).
- Essential tremor.
- Vascular disease (in old age choreiform movements and hemiballismus are usually due to stroke).
- Epilepsy.
- Cerebellar disease.
Aetiological Classification
Vascular Disease
See Chapter 7 for stroke and multi-infarct disease.
Trauma
Fractured Skull
Depressed fractures are important: pieces of bone may damage underlying cortex. Diplopia may indicate a fractured orbit. Fractures through a sinus or the ear may allow entry of organisms and lead to meningitis (prophylactic antibiotics are no longer recommended). Fracture through the temporal bone can result in an extradural haemorrhage. However, a routine X-ray of the skull after an uncomplicated fall is not justified. If an X-ray is done, fractures are hard to spot, but always look for a horizontal line indicating an air/fluid (blood) level.
Subdural Haematoma
This is more common in old age because of increased frequency of falls and it is said that cerebral atrophy allows continued oozing of blood into the subdural space. A subdural may be asymptomatic, cause mild unilateral weakness, intellectual impairment, fits or loss of consciousness. A fluctuating course or disproportionate drowsiness in a patient with a hemiparesis may alert the clinician to this diagnosis. Increased use of anticoagulants in old age (e.g. AF) exposes more patients to the risk of subdural bleeding, especially if prone to fall or drinking to excess, or INR control is poor due to frequent changes in drug regime or poor compliance.
Diagnosis is confirmed by head CT. The hardest decision is whether to operate, and although the appearance of the blood alters with time, it can be hard to be precise about when the subdural haematoma developed. It is difficult to predict whether drainage will improve the clinical state, particularly in dementia. Find out as much as possible about the patient’s prior functional performance from carers or relatives and discuss with a neurosurgeon.
Cord Compression
Cord compression may be secondary to a prolapsed disc, pressure from tumour, osteophytes (especially cervical spondylosis) or collapsed vertebra (usually metastatic disease especially prostate cancer rather than osteoporosis), discitis (vertebral osteomyelitis) or an epidural abscess. A fall may be the precipitating event in a patient who had asymptomatic pathology. Plain X-rays are sometimes difficult to interpret, especially in the cervical spine, where degenerative changes are very common.
The motor effect of a cord lesion depends on the level. A very high neck lesion gives upper motor-neuron (UMN) signs in the arms and legs (spastic quadriparesis). Lower in the neck, compression leads to nerve root or lower motor-neuron (LMN) symptoms and signs in the arms but UMN changes in legs. A thoracic lesion will lead to a spastic paraparesis with UMN signs in the legs. A sensory level, if present, will help identify the region for further investigation; it is usually several segments below the level of cord compression. Patients with cervical and thoracic lesions often have an irritable bladder due to loss of supraspinal inhibition. Remember that the cord ends at L1/2 and the spinal roots (cauda equina) then continue down the spinal canal to their exit foramina. Lumbo-sacral lesions present with LMN signs and sensory loss. Urinary retention is common as the bladder does not empty. Check for anal tone and saddle anaesthesia (use a neurotip in the perianal area).
Sudden onset of compression of the cord or cauda equina is an emergency and rapid investigation is essential if active intervention (surgery or radiotherapy) is to avoid permanent damage. MRI is better than CT, but the latter may be more available.
Lumbar Canal Stenosis
This is usually due to a congenitally narrow spinal canal but presents in middle or old age when osteophytes or a disc encroach on the cauda equina. It may present with weak legs or intermittent pain in the buttocks and legs on walking. It can be distinguished from intermittent claudication (peripheral vascular disease) as patients find climbing stairs easier than walking on the flat (the spine is flexed), the discomfort takes longer – around 10 min – to improve with rest and there may be sensorimotor signs.
Normal Pressure Hydrocephalus (NPH)
NPH is exclusive to later life. Its cause is unknown, but it is assumed that when the condition is developing, abnormal CSF flow must at least sporadically increase the pressure. Its presentation is insidious, with a triad of intellectual failure, unsteadiness (with broad-based gait or gait apraxia) and early urinary incontinence. Diagnosis is made by CT, which will show enlarged ventricles without widened sulci. However, variation in the relative amounts of ventricular enlargement to cerebral atrophy in normal ageing and dementia make this a difficult diagnosis. By the time of diagnosis, the CSF pressure is ‘normal’. Treatment by shunting may be successful. In specialist centres, external lumbar CSF drainage and flow studies are used to try to improve prediction of outcome.
The likelihood of identifying treatable NPH is low. Of 560 cases of dementia seen at the Mayo Clinic from 1990 to 1994, 1% had suspected NPH, but none of the 3/5 treated with ventriculoperitoneal shunting improved. In a small series with functional MRI before and after CSF drainage, motor function improved but cognition did not. Where there is benefit, subjects with short duration gait problems do best. A meta-analysis found that the mean rate of shunt complications (including death, infection, seizures, shunt malfunction and subdural haemorrhage) was 38%. Enthusiastic neurosurgeons continue to offer shunting and report good outcome data, but there is only one trial identified in PubMed and it is not clear if patients were randomized. Cochrane concludes that there is no evidence to support shunting.
Hydrocephalus may also be secondary to previous cerebral damage from episodes of bleeding (especially SAH) or meningitis. A strategically placed space-occupying lesion in the mid-brain may also lead to hydrocephalus.
Degenerative or Idiopathic Disease
Parkinson’s Disease (PD)
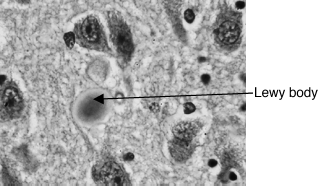
This is an idiopathic degenerative condition with progressive death of the dopaminergic neurons of the substantia nigra (SN) in the basal ganglia. Symptoms appear when around 80% of the dopamine has been lost and are due to a lack of dopamine and a relative excess of acetylcholine. PD is thought to occur in the genetically susceptible exposed to an environmental trigger, but the nature of both components remains unknown (chronic low-dose pesticide exposure remains an environmental favourite). Rare young-onset PD has a clearer genetic component and some of the gene defects are characterized. The post mortem finding of Lewy bodies (intracytoplasmic inclusion bodies containing alpha synuclein) restricted to the SN is pathognomonic (Figure 8.1). Many cases diagnosed in life, even by experts (perhaps 10%), are not confirmed as PD on post-mortem examination. The incidence increases with age (250 in 105 aged 60–69 to 2,000 in 105 aged over 80). Patients can be encouraged to donate their brain to the PD brain bank.
Figure 8.1 Immunohistochemistry for alpha-synuclein showing positive staining of an intraneuronal Lewy-body in the substantia nigra in Parkinson’s disease (Marvin, http://commons.wikimedia.org/wiki/File:Lewy_Body_alphaSynuclein.jpg.)
Diagnosis
PD is always a difficult diagnosis in the early stages, especially in the very old, who may ‘normally’ have some features of extrapyramidal rigidity. The triad of classical symptoms and signs are poverty of movement (akinesia, bradykinesia), regular tremor at rest (5/sec, ‘pill rolling’) and rigidity of extrapyramidal type (‘lead pipe’) or cogwheel rigidity in the presence of tremor.
The tremor may be obvious, is usually a rest tremor and may be unilateral. It disappears in sleep. The bradykinesia may be apparent as paucity of facial expression (Parkinsonian facies) and fine movements are difficult, typically doing up buttons. Handwriting may get smaller during the course of a sentence (micrographia). Speech is soft (dysphonic), monotonous and becomes dysarthric. The stiffness may be misinterpreted as arthritis, and although PD does not affect the sensory system, the joint stiffness, particularly in bed at night, may be painful. The gait is characteristic with a flexed posture, tendency to shuffle, loss of arm swing and impaired postural reflexes, which make the patient likely to fall. Stopping, starting and turning pose most difficulty, and if a walking frame is needed, the wheeled type is usually recommended. As the disease progresses, constipation, bladder instability and drooling may be troublesome.
Documented response to therapy may be helpful in confirming the diagnosis, e.g. measuring the time to walk a set distance (10 m) or to carry out a tap test – the number of pronations and supinations the patient can achieve in a minute, tapping on a desk, or inspection of handwriting (micrographia should be seen to improve). Diagnosis is clinical, but a 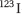 -FP-CIT-SPECT [single-photon emission computed tomography (SPECT) scan, which uses a cocaine analogue to image dopamine transporter receptors on the presynaptic nigrostriatal terminals, can distinguish PD (where the cells die so the amount of receptor falls) from essential tremor (appearance resembles healthy controls).
-FP-CIT-SPECT [single-photon emission computed tomography (SPECT) scan, which uses a cocaine analogue to image dopamine transporter receptors on the presynaptic nigrostriatal terminals, can distinguish PD (where the cells die so the amount of receptor falls) from essential tremor (appearance resembles healthy controls).
Management
All patients with PD benefit from a multidisciplinary package of care of which drug treatment is only one component. As the disease progresses, the relative emphasis of the components will change. Learn the following list – suitably modified, it will provide an outline of how to manage most chronic conditions at any age, from MS to COPD!
Management options include:
- Physiotherapy: work on posture, gait and falls prevention.
- Occupational therapy: maintaining skills, home modification, etc.
- Speech and language therapy: speech, facial expression, swallowing.
Table 8.1 Drug management of Parkinson’s disease
| Mechanism | Name | Prescription tips (see BNF section 4.9) |
| Replenish striatal dopamine | Levodopa with peripheral dopa-decarboxylase inhibitor as Sinemet® (co-careldopa) or Madopar® (co-beneldopa) | Start low, increase slowly balancing response with side-effects, with meals initially to reduce nausea, later before meals as drug competes for absorption with amino acids from a protein meal. Can use slow-release preparation from the start (but little benefit) or to cover the night; dispersible preparation if swallowing a problem. About 85% of patients respond to levodopa |
| Duodopa® | Direct duodenal infusion of levodopa (see text) | |
| Catechol-O- methyltransferase inhibitor (COMTI) | Entacapone, tolcapone | Entacapone, used with levodopa to reduce end of dose deterioration, may colour the urine red. Tolcapone only used if entacapone unsuitable as tolcapone occasionally causes severe hepatotoxicity |
| Monoamine-oxidase-B inhibitor (MAO-BI) | Selegiline, rasagiline | Used as early monotherapy or with levodopa to reduce end of dose deterioration. Concern (inconclusive) that selegiline increased mortality led to sublingual preparation to avoid first-pass, reducing amphetamine-related metabolites. Give in the morning as a mild stimulant. |
| Rasagiline, once a day, may be better as metabolites are not amphetamines | ||
| Dopamine agonists | Increase dose slowly as hypotension can occur in the first few days. The therapeutic effect is mediated via the D2 receptor; other effects depend partly on their activity at other dopaminergic receptors and whether they are derived from ergot | |
| Ergoline family: | Rarely used in new patients | |
| • lisuride | ||
| • pergolide | ||
| • cabergoline | ||
| Non-ergoline: | Ropinerole and pramipexole licensed for monotherapy or with levodopa. Eye checks recommended with pramipexole so ropinerole gaining market share. Rotigotine patch for monotherapy in early PD. Apomorphine used subcutaneously; via a pen or pump under specialist supervision for intractable fluctuations | |
| • ropinerole | ||
| • pramipexole | ||
| • rotigotine | ||
| • apomorphine | ||
| Antimuscarinic drugs | Orphenadrine, benzatropine, procyclidine, trihexphenidyl | Little to choose between drugs. Rarely used in elderly as worsen cognition, GI side-effects and urine retention. Used for drug-induced PD, tremor and may help drooling |
Drugs
Research is aimed at finding ‘neuroprotective’ drugs that prevent cell death. Increasing evidence suggests that in PD neurons die by apoptosis, which may be triggered by mitochondrial impairment and oxidative stress. Selegiline was thought to be neuroprotective but this is not the case. Several drugs now in trials have looked promising in animal studies. There is some evidence for benefit of co-enzyme Q10, a supplement available in health shops, but larger trials are needed.
Current drug treatment aims to restore transmitter balance in the basal ganglia. Whilst the classes of drugs available are logical (Figure 8.2), the order in which they are used varies not only with the patient but also with the prescriber, i.e. the weight of evidence does not clearly support one course of action. Details of drugs used in the management of PD are given in Table 8.1. Assuming the diagnosis is correct, levodopa preparations usually provide excellent benefits initially, but over a few years the ‘long-term levodopa syndrome’ emerges. Problems may be predictable at first. For example, soon after a dose there may be involuntary movements (peak-dose dyskinesia), and as the next dose becomes due, the effect of the previous dose may wear off so that the patient becomes rigid, immobile and frozen or ‘off’. These effects can be ameliorated by careful juggling of doses and timing, but eventually the fluctuations can become severe, apparently random and the patient may alternate between being ‘on’ for short periods only, with ‘offs’ and disabling dyskinesias. There is some evidence that the duration or dose × years of levodopa treatment affects the development of this syndrome, perhaps because of pulsatile stimulation of the receptors. Long-acting agonists are another option for initial treatment and may be favoured in younger patients. There are two groups of agonists, ergot and non-ergot derived, but a range of emerging and serious idiosyncratic reactions have almost stopped new prescriptions of the ergot family. In frail older people, if PD is already impacting on function, many doctors start with levodopa. In this group, the dose of levodopa is often limited by neuropsychiatric problems (confusion, hallucinations). Younger patients tolerate bigger doses without confusion, but dyskinesias tend to become more troublesome with time. The development of a transdermal drug delivery system is an attractive option. However, local skin reactions are common with rotigotine, the first patch to be available.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree