7.1
Introduction
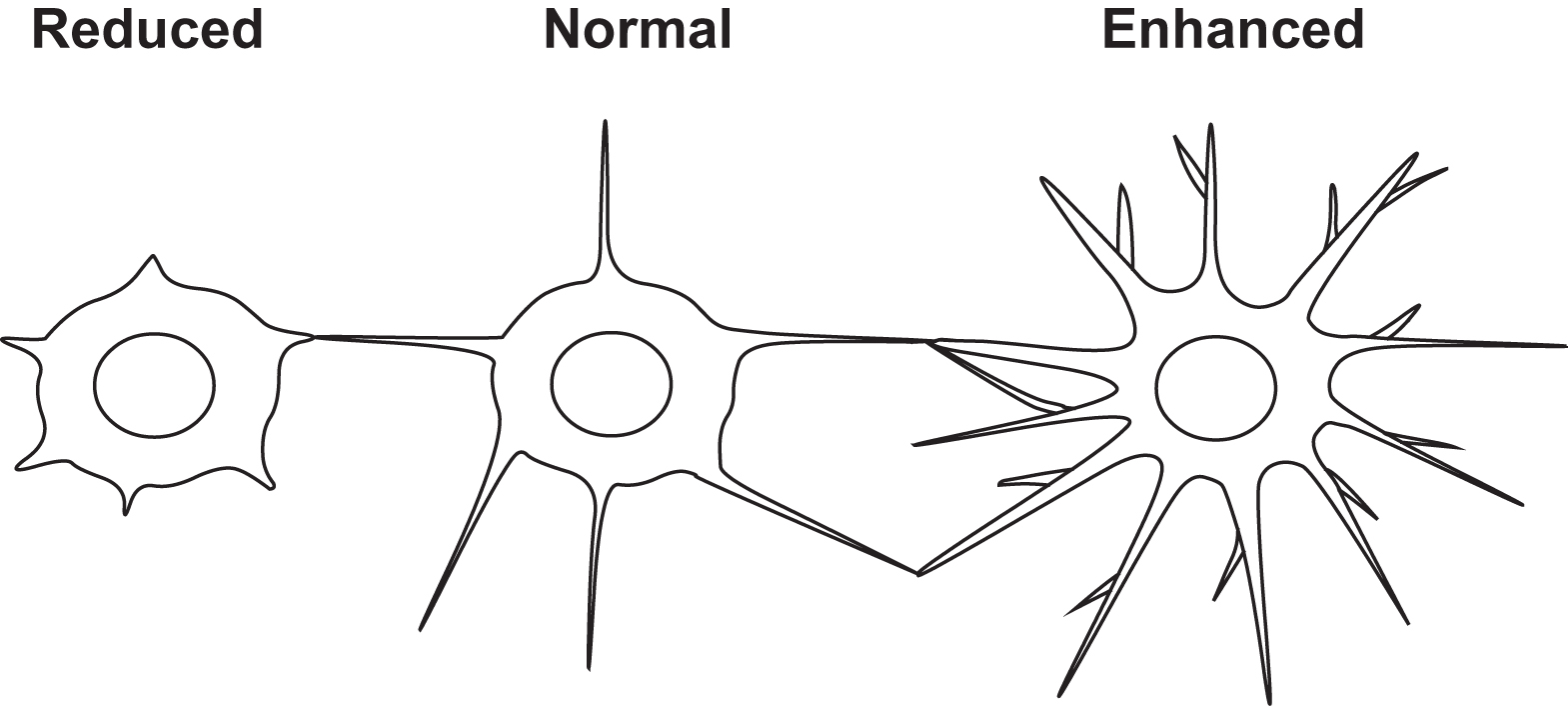
Osteocytes are defined as cells embedded in the mineralized bone matrix and compose over 90%–95% of all bone cells . They are regularly dispersed throughout the mineralized matrix, connected to each other and cells on the bone surface through slender, cytoplasmic processes radiating in all directions but generally perpendicular to the bone surface ( Fig. 7.1 ). The cell processes or dendrites pass through the bone in thin canals, called canaliculi, which connect osteocytes with cells on the bone surface. These cells are defined by their location, not by their function as is the case for osteoblasts and osteoclasts. This lack of a functional definition implied a lack of knowledge of function. Since this chapter was first written in 2005, a virtual explosion of data regarding osteocyte function has occurred. The earliest proposed major function of osteocytes was to translate mechanical strain into biochemical signals between osteocytes and cells on the bone surface to affect (re)modeling. Osteocytes were thought to respond to mechanical strain to send signals to stimulate resorption or formation . Not only do these cells communicate with each other and with cells on the bone surface, but their dendritic processes are in contact with the bone marrow and the vasculature . Multiple connections through the tips of their dendritic processes imply that osteocytes function as “communicators.” Not only do these cells function as mechanosensors, communicators and orchestrators of bone modeling and remodeling but also as regulators of calcium and phosphate homeostasis and function as endocrine cells sending signals to distant tissues . These cells have now proven to be multifunctional as outlined in this chapter.

7.2
Osteocyte ontogeny
Osteoprogenitor cells residing in the bone marrow give rise to osteoblasts that progress through a series of maturational stages resulting in the mature osteocyte. Biomarkers and functional assays have been used to discriminate between these various stages. Although numerous markers for osteoblasts are available (cbfa1, osterix, alkaline phosphatase, collagen type 1, osteocalcin, etc., see also Chapter 3: Development of the skeleton ), only within the past decade have markers have been available for osteocytes. Osteocytes not only share some markers with their progenitors-osteoblasts but also express unique markers based on their morphology and potential function. Markers for osteocytes include phosphate-regulating neutral endopeptidase on the chromosome X (Phex), Dentin Matrix protein 1 (Dmp1), and E11/gp38 for early osteocytes and sclerostin, fibroblast growth factor 23 (Fgf23), and matrix extracellular phosphoglycoprotein (MEPE) for mature osteocytes. These and other markers will be defined later in this chapter.
Kalajzic et al. have used promoters for osteocalcin and collagen type 1 linked to reporters such as green fluorescent protein (GFP) to examine transgene expression during osteoblast differentiation . Osteocalcin-GFP was expressed in a few osteoblastic cells lining the endosteal bone surface and in scattered osteocytes, whereas GFP driven by the collagen type 1 promoter was strongly expressed in osteoblasts and osteocytes. These investigators also generated an osteocyte-selective promoter, the 8 kb Dmp1 driving GFP that showed expression in osteocytes . This mouse model has proved useful to examine osteocyte ontogeny and to determine osteocyte function. However, there remains a need to identify and generate additional osteocyte promoters not only to drive reporters but also to drive Cre expression.
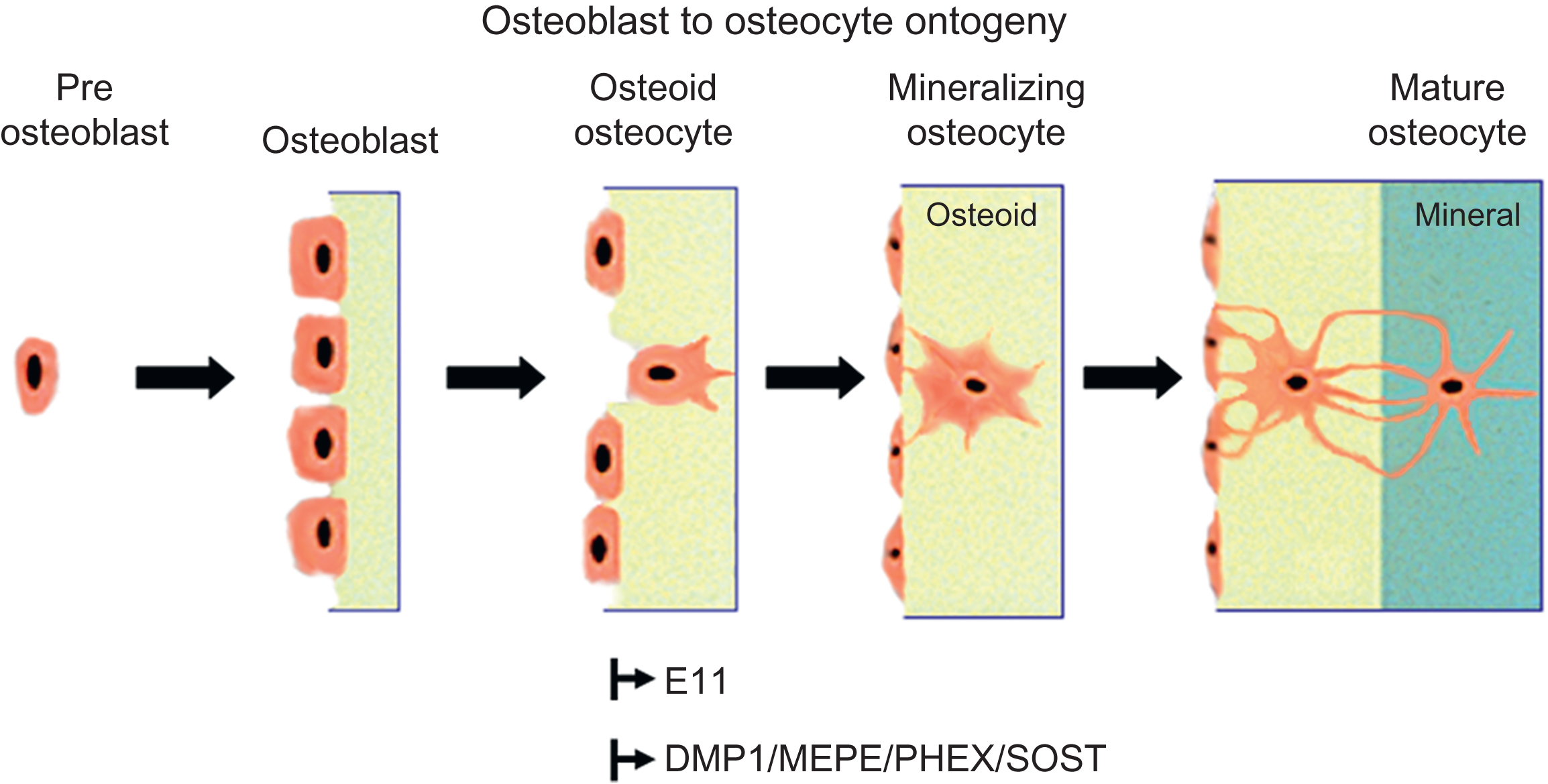
The differentiating osteoblast has one of three fates; it can become embedded in its own osteoid and continue differentiation into an osteocyte, it can quiesce into a lining cell; or, more likely, it can undergo apoptosis (for review see Manolagas ). Karsdal et al. proposed that matrix metalloproteinase activation of latent transforming growth factor β (TGFβ) blocks osteoblast apoptosis thereby delaying differentiation into osteocytes . Identification of mechanisms responsible for osteoblast apoptosis has implications for the development of strategies that could potentially increase bone mass. Inhibition of osteocyte apoptosis may have beneficial or nonbeneficial effects on bone depending on condition as addressed later in this chapter ( Fig. 7.2 ).
7.3
Osteoid–osteocytes
Osteoblasts, osteoid cells and osteocytes may play different roles in the initiation and regulation of mineralization of bone. In 1976 and 1981, Bordier et al. and Nijweide et al. proposed that osteoid–osteocytes play an important role in the initiation and control of mineralization of the bone matrix. Osteoid–osteocytes were described by Palumbo to be cells actively making matrix and calcifying this matrix. Like osteoblasts, their activity is polarized toward the mineralization front to which their cellular processes are oriented, whereas processes oriented toward blood vessels only begin to appear when mineralization begins to spread around the cell. The cell body reduces in size in parallel with the formation of cytoplasmic processes with a reduction of about 30% at the osteoid–osteocyte stage and 70% with complete maturation of the osteocyte. During the time it takes for an osteoblast to become an osteocyte, the cell has manufactured three times its own volume in matrix . For a review of osteoblast to osteocyte transformation, see Refs. . These authors suggest that once a cell is surrounded by osteoid the differentiation process does not end, but should be viewed as a continuum.
Mikuni-Takagaki et al. proposed that casein kinase II, produced in high amounts by embedding osteoid–osteocytes and not by osteoblasts, is responsible for the phosphorylation of matrix proteins essential for mineralization . Phosphoproteins appear to be essential for bone mineralization as evidenced by in vitro crystal nucleation assays and in vivo by osteomalacia in animal models with deletion of specific genes such as Dmp1 and Phex . Deletion of inhibitors of mineralization such as osteoblast/osteocyte factor 45/MEPE, results in osteopetrosis . These phosphoproteins are expressed late in osteoblast differentiation and are all molecules that are highly expressed in osteoid–osteocytes. Therefore the embedding osteoid cell and the osteocyte play roles in the mineralization process and in phosphate metabolism (see later).
7.4
Osteocyte-selective genes/proteins and their potential functions
Although osteoblasts have been described to have several markers such as runx2, osterix, alkaline phosphatase, collagen type 1, and osteocalcin, new markers have been described for osteocytes. Early, initial markers for osteocytes ranged from low alkaline phosphatase to high casein kinase and osteocalcin protein expression, compared to osteoblasts . Antigens such as E11/gp38/podoplanin have been identified that are more highly expressed specific for early embedding osteocytes compared to osteoblasts, and antigens such as Phex, Dmp1, MEPE, sclerostin, ORP150, and Fibroblast growth factor (FGF23) have been found to be more highly expressed in osteocytes compared to osteoblasts as discussed later. Franz-Odendaal et al. provide a list of molecular markers for the preosteoblast to the osteocyte .
E11 is the name given to a molecule that is expressed in early osteocytes and found on the dendritic processes of osteocytes, but not osteoblasts in vivo and cementocytes . The major function of E11 may be in the formation of dendritic processes as reduction in protein expression led to a decrease in dendrite extension in MLO-Y4 osteocyte-like cells and overexpression in an osteoblast-like cell line led to the generation of extended cytoplasmic processes . The molecule colocalizes with ezrin, radixin, and moesin, abbreviated ERM , proteins that are concentrated in cell-surface projections where they link the actin cytoskeleton to plasma membrane proteins. ERMs play structural roles and are involved in cell motility . E11 was also found to be physically associated with CD44 in tumor vascular endothelial cells and as CD44 is highly expressed in osteocytes compared to osteoblasts this suggests that E11 associates with CD44 and the ERMs to induce and regulate the formation of dendritic processes in osteoid–osteocytes and osteocytes, but this has yet to be proven.
CD44 is a major component of the osteocyte pericellular matrix and is more highly expressed in osteocytes than osteoblasts . CD44 is a membrane bound protein and hyaluronic acid receptor that interacts with the ERMs. Not only has CD44s been shown to associate with E11 but also with osteopontin , another member of the SIBLING (Small, Integrin-Binding Ligand, N-linked Glycoprotein) family, suggesting that other members of this family such as Dmp1 and MEPE may also interact with CD44.
Proteomic studies have also been used to identify proteins that are highly expressed by osteocytes as compared to osteoblasts. The expression of ORP150, Destrin and Macrophage-capping protein (CAPG) were found to be increased in MLO-Y4 cells and osteocytes in vivo, relative to MC3T3 preosteoblasts and osteoblasts respectively . Destrin and CAPG are believed to play a role in dendrite formation, whereas ORP150 is thought to protect the cells from the hypoxic conditions encountered within the mineralized bone matrix.
Nijweide et al. found that their osteocyte-specific antibody, Mab OB7.3 recognizes Phex . This antibody allowed them to purify avian osteocytes from enzymatically isolated bone cells for subsequent study. Phex was originally described on the plasma membrane of osteoblasts and osteocytes and loss of function mutations in this gene result in X-linked hypophosphatemic rickets . PHEX is a metalloendoproteinase that plays a role in phosphate homeostasis and bone mineralization. These investigators propose that the osteocyte network may be considered to be an endocrine gland that regulates bone phosphate metabolism through expression of PHEX. Loss of PHEX expression, or mutations such as in the X-linked rickets mouse model, the Hyp mouse, lead to increased levels of FGF23 responsible for hypophosphatemia . FGF23 is not normally expressed at high levels in osteocytes in the healthy state but its expression in osteocytes is dramatically elevated in both PHEX- and DMP1-associated hypophosphatemic rickets . Osteocytes appear to be the main source of the elevated circulating levels of FGF23 seen in these mouse models, giving support to the notion that osteocytes also act as endocrine cells.
Feng et al. found the DMP1 gene expressed in early embryonic bone development in hypertrophic chondrocytes and osteoblasts and later during postnatal bone formation where it is highly expressed in osteocytes, consistent with the observations of Toyosawa who observed high expression in osteocytes, but not in osteoblasts . DMP1 is specifically expressed along and in the canaliculi of osteocytes within the bone matrix . The function of DMP1 in osteocytes may be related to the posttranslational processing and modifications of the protein as a highly phosphorylated protein and regulator of hydroxyapatite formation . Deletion of this gene in mice results in a phenotype similar if not identical to the Hyp phenotype , suggesting that DMP1 and PHEX are interactive and essential for phosphate metabolism. Both genes appear to downregulate FGF23.
Osteoblast/osteocyte factor 45 (OF45) also known as MEPE is also highly expressed in osteocytes as compared to osteoblasts. MEPE was isolated and cloned from a tumor-induced osteomalacia (TIO) tumor cDNA library . Independently, others isolated and cloned the rat and mouse homologues based on the ability of MEPE to regulate mineralization . The MEPE protein is highly phosphorylated in a region called the ASARM region. Cathepsin D or B can cleave MEPE, releasing the C-terminal phosphoprotein region, and this C-terminal ASARM region is a potent inhibitor of mineralization in vitro . High ASARM peptide production by osteocytes supports the hypothesis that ASARM peptide may play a role in osteomalacia such as that observed in the Hyp mouse model. Messenger RNA expression for OF45/MEPE begins at E20 in more differentiated osteoblasts that have become encapsulated by bone matrix . These authors place the sequence of expression of osteoblast to osteocyte transition markers as osteocalcin during encapsulation, followed by DMP1, followed by OF45 as a marker of the mature osteocyte. Deletion of this gene in mice results in increased bone formation and bone mass and resistance to age-associated trabecular bone loss . The authors speculate that as terminally differentiated osteoblasts become embedded in the bone matrix, OF45 expression is increased and maintained in mature osteocytes and that osteocytes act directly on osteoblasts through OF45 to inhibit their bone-forming activity. Interestingly, DMP1 and OF45/MEPE belong to the SIBLING family that also includes bone sialoprotein, osteopontin, and sialophosphoprotein . This family of proteins may function differently in osteocytes compared to other cell types especially upon phosphorylation with casein kinase.
The SOST gene encodes a protein, sclerostin, that is highly expressed in osteocytes and inhibits bone formation . The human condition of sclerosteosis is due to a premature termination of the SOST gene . Transgenic mice lacking sclerostin have increased bone mass. Controversy exists as to whether sclerostin is a BMP antagonist or functions as a Wnt antagonist . Sclerostin appears to be an antagonist of Lrp5, a gene shown to be important as a positive regulator of bone mass . It is suggested that sclerostin may be transported through osteocyte canaliculi to the bone surface to inhibit bone-forming osteoblasts. It has also been proposed that the anabolic effects of PTH are through inhibition of SOST expression . Sclerostin expression is decreased by mechanical loading and it was recently reported that the anabolic effects of loading are dependent on this decrease in expression . Sclerostin has also recently been implicated as a regulator of the late osteoblast differentiation to the preosteocyte through its regulation of PHEX and MEPE . The profound gain of bone mass observed after the deletion of Sost has led to the development of monoclonal antibodies directed against sclerostin as a potential anabolic treatment for osteoporosis. Animal studies and early results from clinical trials suggest that these antibodies may be an effective therapeutic for increasing bone mass and for acceleration of fracture healing , as will be discussed later.
Osteocytes have also been found to be intensively immunoreactive for neurokinin-1 whereas lining cells were found to be positive for neurokinin-2 . Neurokinin-1 and neurokinin-2 are tachykinin receptors for neuropeptides. The presence of these receptors suggests that sensory nerves may regulate the function of bone cells. Neuropeptide Y can be modulated by mechanical loading, and osteocyte-expressed Neuropeptide Y can inhibit osteoblast activity and differentiation . For additional hypotheses concerning the possible relationship of the neural system to bone see review by Turner .
As will be discussed later in the section on osteocyte modification of their microenvironment, osteocytes can also express “osteoclast-specific genes.” With lactation osteocytes can express the “machinery” to remove their perilacunar matrix that includes genes such as tartrate resistant acid phosphatase (TRAP), cathepsin K, carbonic anhydrase 1 and 2, matrix metallopeptidase 13 (MMP13), and the ATPase transporter subunit, ATP6V9D2 .
7.5
Morphology of osteocytes: lacunocanalicular system and dendrite formation
The transformation of a plump polygonal osteoblast to a dendritic osteocyte is striking and dramatic and clearly requires extensive reorganization of the cytoskeleton. The osteocyte loses the typical apical and basolateral plasma membrane polarization characteristic of osteoblasts . Actin filaments were found to be crucial for the maintenance of the osteocyte processes and two actin-bundling proteins, alpha-actinin, and fimbrin were shown to be useful as markers for osteocytes . Stronger signals of fimbrin were observed at branching points in dendrites. Villin, another actin-bundling protein, is also higher in osteocytes than osteoblasts. Staining patterns were distinct between osteoblasts and osteocytes with filamin along stress fibers in osteoblasts, but only at the base of processes in osteocytes. Staining for spectrin was punctate in osteoblasts but filamentous in osteocytes .
As discussed earlier, a hydrophobic membrane protein called E11 appears to play a role in dendrite formation. Although known as E11 in osteocytes, it is known by other names (gp38/podoplanin/T1alpha) in other cell types (endothelial cells/podocytes in kidney/type 2 alveolar lung cells). The earliest description of the gene for E11 was in 1990 as a previously unknown phorbol ester inducible gene in MC3T3 osteoblast-like cells, called OTS-8 . A common feature of virtually all cell types that express E11 is their extended cytoplasm or dendritic nature. The fact that E11 is often found in cells that are exposed to an external or internal fluid compartment and is highly negatively charged and resistant to proteases suggests that the molecule provides a physical barrier that plays a role in protecting cells. Deletion of E11 results in mice that die at birth due to respiratory failure as a result of failure of type 2 alveolar lung cells to differentiate into type 1 alveolar lung cells . A potential function for E11 in osteocytes was shown by reducing its protein expression using an siRNA approach that prevented dendrite elongation in MLO-Y4 cells in response to shear stress . Also as mentioned earlier, osteocytes embedding into osteoid expresses greater amounts of destrin and CapG, which is thought to play a role in actin filament reorganization, than osteoblasts on the bone surface . Paic et al. showed that the actin binding protein, Capzb, is upregulated in osteocytes together with several genes related to muscle contractility, such as myosin heavy and light chains, α-actin, troponins, tropomyosins, and α-actinin . Both CapG and Capzb belong to the gelsolin family of proteins, which regulate the length of actin filaments by capping their barbed positive ends. Destrin is a member of the actin depolymerizing factor/cofilin family and regulates actin assembly and disassembly. These proteins control the dynamics of actin polymerization and depolymerization.
Osteocytogenesis has been thought to be a process whereby some osteoblasts become encased in osteoid that passively mineralizes. The embedded cell does not participate in the embedding or mineralization process but is acted upon by surface cells. However, Holmbeck et al. have shown osteocytogenesis to be an active invasive process requiring cleavage of collagen and potentially other matrix molecules. Osteocytes in mice null for the metalloproteinase MT1-MMP have significantly reduced number and length of dendritic processes. MT1-MMP is a membrane-anchored proteinase that can cleave collagens type 1, 2, and 3, fibrin, fibronectin, and other matrix molecules. In this mouse model the almost complete lack of dendritic processes did not appear to affect viability or density of osteocytes. This is in contrast to studies by Zhao et al. where osteocytes in a mouse model of collagenase resistant type 1 collagen did show increased apoptosis. However, in the MT1-MMP null mouse it is difficult to determine the effect of a lack of dendritic processes on either osteocyte function or effects on the skeleton as this mouse exhibits multiple defects, such as dwarfism, due to a lack of MT1-MMP in other skeletal tissues . Interestingly, these investigators and others have shown an age-dependent increase in the number of canaliculi suggesting: (1) that new bone made in the adult or aging animals generates osteocytes with more canaliculi or (2) that the embedded osteocyte can generate new dendrites ( Fig. 7.3 ).
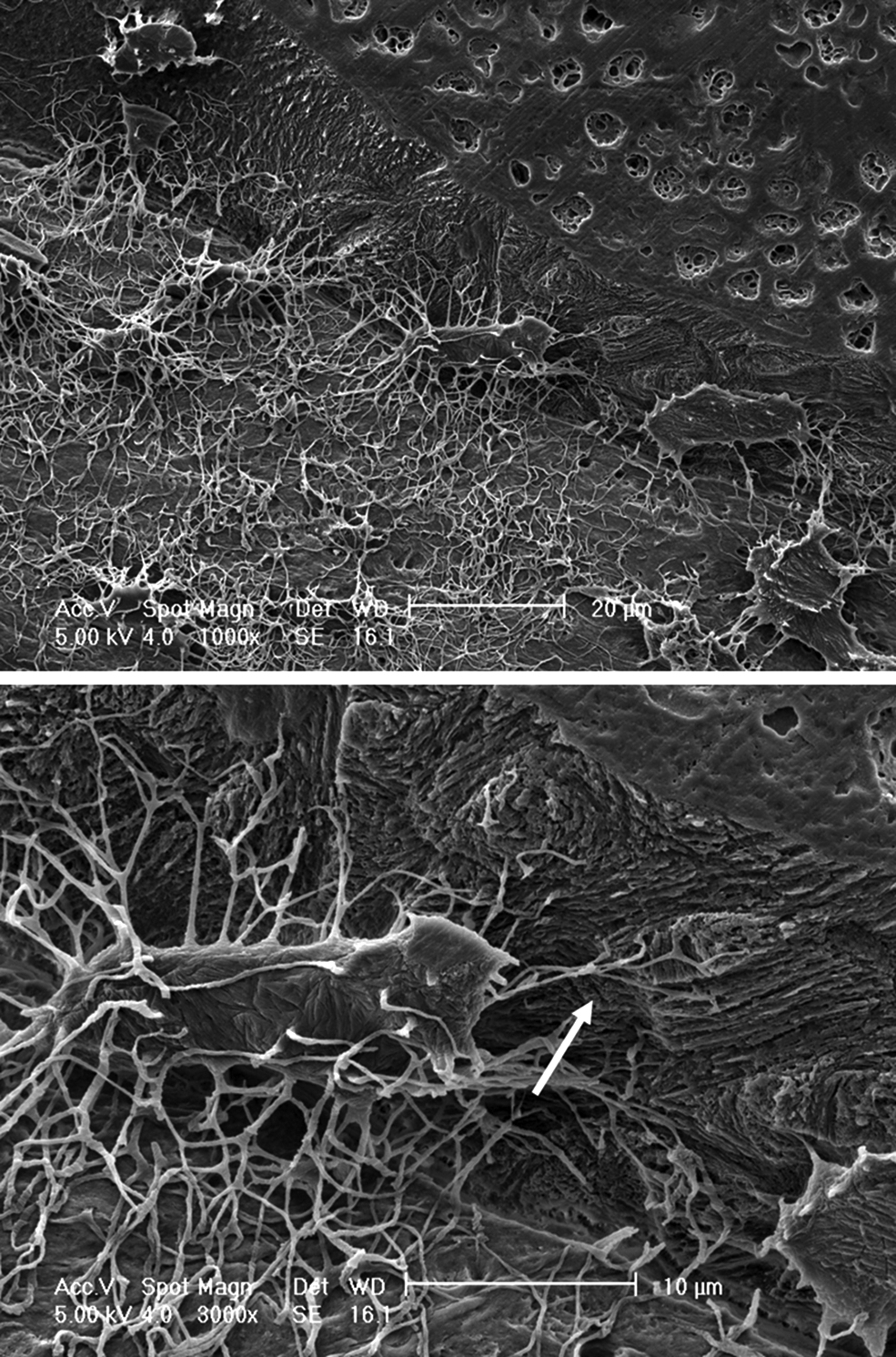
The osteocyte has been viewed as a quiescent cell type. However, evidence is accumulating that these cells are more active than previously known. Dallas et al. used calvarial explants from transgenic mice with GFP expression targeted to osteocytes and time lapse dynamic imaging to observe living osteocytes within their lacunae . Surprisingly, these studies have revealed that, far from being static, the osteocyte may be highly dynamic. Embedded osteocytes expand and contract their cell body within the boundaries of their lacunae and extend and retract their dendrites over a 24 hour period. These data suggest that dendrites, rather than being permanent connections between osteocytes and between osteocytes and surface cells, may be dynamic structures that can be altered in response to stimuli.
7.6
Osteocyte cell models
There are several reasons why much less is known concerning osteocyte function compared to osteoblasts and osteoclasts. These include the following: (1) it is difficult to isolate sufficient numbers of osteocytes from the mineralized bone matrix for many types of studies, (2) it is difficult to maintain their differentiated function in vitro, (3) there was a lack of suitable cell lines, and (4) there was a lack of availability of osteocyte-specific promoters for targeted transgenic approaches. Primary cultures of osteocyte-like cells can be prepared by sequential alternating digestions of fetal rat and chick calvaria with collagenase and EDTA . Cells removed in early digests are fibroblasts/osteoblasts and those released later represent a population enriched for osteocytes. An osteocyte-specific antibody for avian osteocytes, Mab OB7.3, which recognizes Phex was successfully used in antibody panning techniques to obtain an essentially pure population of avian osteocytes . Each of these isolation procedures works best with young or hypomineralized bone but not with adult or aged hypermineralized bone. Stern et al. have developed an approach where hypermineralized bone particles can be generated to yield an osteocyte population that can be used for experiments . These primary osteocyte culture systems have been useful in beginning to define the properties of these cells and investigate their biochemistry. Mice in which the 8 kb DMP1 promoter drives GFP expression can be used to study osteocytes especially in conjunction with fluorescence-activated cell sorting to obtain a highly purified population.
However, the yields of primary osteocytes are low, thereby making it difficult to obtain large enough numbers of cells for detailed or extensive biochemical studies. To compensate for these difficulties, investigators have attempted to make osteocyte cell lines. To date, there are six models with osteocyte-like characteristics: HOP-01-C1, MLO-Y4, MLO-A5, IDG-SW3, OCY454, and OMGFP66. An early model of the pre- or early osteocyte is the HOB-01-C1 human bone cell line , a temperature sensitive line that proliferates at 34°C and stops growing at 39°C. They have cellular processes, low alkaline phosphatase, high osteocalcin, and high CD44 expression.
An early model for murine early osteocytes is the MLO-Y4 osteocyte-like cell line that has been used extensively to investigate osteocyte function . This cell line was derived from a transgenic mouse in which the immortalizing T-antigen was expressed under the control of the osteocalcin promoter. MLO-Y4 cells exhibit the properties of osteocytes including high expression of osteocalcin, low expression of alkaline phosphatase, high expression of connexin 43, and the antigen E11, a known marker of osteocytes. MLO-Y4 cells retain a dendritic morphology, similar to that observed in primary osteocyte cultures. Numerous laboratories have used this cell line to investigate osteocyte cell function, especially gap junctions, hemichannels, mechanosensitivity, and others.
The MLO-A5 is postosteoblast/preosteocyte-like cell line, established from the long bones of 14-day-old mice and expressing the large T-antigen driven by the osteocalcin promoter, differentiates into osteoid–osteocyte-like cells . These cells will mineralize in the absence of β-glycerolphosphate in 6–7 days in sheets, not nodules, and this process is accelerated by the addition of an external source of phosphate. Fourier transform infrared spectra of these cultures are very similar to those in normal bone showing that this cell line reproduces primary mineralization . MLO-A5 cells express all of the markers of the late osteoblast such as high alkaline phosphatase, bone sialoprotein, PTH type 1 receptor, and osteocalcin. In culture, these cells begin to express markers of osteocytes as they generate cell processes. These cells generate nanospherulites that mineralize while budding from their developing cellular processes that become associated with and initiate collagen mediated mineralization . These cells have been useful for studies that require a mineralizing cell.
The IDG-SW3 cell line differentiates from the late osteoblast to the late osteocyte . The cell line was created by crossing the 8 kb Dmp1-GFP transgenic mouse line with the Immortomouse. This mouse carries a γ-IFN-inducible promoter driving expression of a thermolabile large T-antigen ( H-2K b – tsA58), enabling conditionally immortalization of cells derived from their tissues. GFP-positive cells were isolated from the long bones of a 3-month-old mouse by fluorescence-activated cell sorting to establish this cell line. These cells express the SV40 T-antigen when cultured at 33°C in the presence of interferon-gamma and proliferate rapidly. However in the absence of interferon-gamma and cultured at 37°C, their proliferation is reduced and the cells undergo differentiation if supplemented with ascorbic acid and β-glycerophosphate. These cells temporally express osteocyte marker genes from the early osteocyte marker E11 to the mature osteocyte marker sclerostin. Similar to osteocytes in vivo, these cells have been shown to increase FGF23 mRNA expression in response to treatment with 1,25-dihydroxyvitamin D 3 and to downregulate Sost expression with PTH treatment . This cell line faithfully recapitulates the differentiation process from osteoblast to late osteocyte as observed in vivo. Another cell line has been generated using the Immortomouse crossed with the 8 kb Dmp1-GFP mouse called Ocy454 reported to express sclerostin at an earlier time in culture than the IDG-SW3 cells.
A new very exciting cell line is the OMGFP66 cell line . This cell line actually forms bone-like structures containing cells that appear identical to primary osteocytes in vivo. This cell line will be a useful addition to the preexisting models for osteocytes.
7.7
Mechanisms and response of osteocytes to mechanical forces
A known key regulator of osteoblast and osteoclast activity in bone is mechanical strain. Under normal conditions, bone formation and resorption are balanced to maintain bone mass. However, by the process of adaptive remodeling, the skeleton is able to continually adapt to its mechanical environment by adding new bone to withstand increased amounts of loading and removing bone in response to unloading or disuse (reviewed in Refs. ). It was actually Galileo in 1638 who is first documented as suggesting that the shape of bones is related to loading. Julius Wolff in 1892 more eloquently suggested that bone accommodates or responds to strain. The cells of bone with the potential for sensing mechanical strain and translating these forces into biochemical signals include bone lining cells, osteoblasts, and osteocytes. Of these, the osteocytes, with their distribution throughout the bone matrix and their high degree of interconnectivity, are thought to be the major cell type responsible for sensing mechanical strain and translating that strain into biochemical signals related to the intensity and distribution of the strain signals . This hypothesis has been supported by studies by Tatsumi et al. who found that mice with targeted deletion of osteocytes using the diphtheria toxin receptor were resistant to unloading-induced bone loss .
Various studies have demonstrated load-related responses in osteocytes, supporting their proposed role as mechanotransducers in bone. Within a few minutes of loading, glucose 6-phosphate dehydrogenase, a marker of cell metabolism, is increased in osteocytes and lining cells . By 2 hours, c-fos mRNA is evident in osteocytes and by 4 hours, TGFβ and insulin-like growth factor 1 mRNAs are increased . The DMP1 gene is activated in response to mechanical loading in osteocytes in the tooth movement model and in the mouse ulna loading model of bone formation . E11 is also increased in response to mechanical load, not only in cells near the bone surface by also in deeply embedded osteocytes . Unloading increases RANKL expression in osteocytes , which may be responsible for the bone loss associated with unloading. Mechanical loading has been shown to reduce sclerostin in the mature osteocytes , whereas hindlimb unloading has been shown to increase sclerostin expression . Deletion of Lrp5, a major coreceptor for Wnt signaling, resulted in mice that showed impaired osteogenic responses to anabolic loading. Deletion of only one allele of β-catenin results in mice with a normal skeleton but a completely abrogated response to anabolic loading . Taking all these studies together, it appears that the components of the β-catenin pathway in osteocytes play an important role in bone responses to loading.
The parameters for inducing bone formation or bone resorption in vivo are fairly well known and well characterized. Bone mass is influenced by peak applied strain as shown by Rubin and Lanyon Bone formation rate is related to loading rate as shown by varying the frequency of applied bending while keeping the magnitude of applied load constant . At bending frequencies of 0.5–2.0 Hz, bone formation rate increased as much as fourfold, while no increase was observed at frequencies lower than 0.5 Hz. When rest periods are inserted, the loaded bone shows increased bone formation rates and mechanical properties when compared to bone subjected to a single bout of mechanical loading . Frequency, intensity, and timing of loading are all important parameters. Improved bone structure and strength is greatest if loading is applied in shorter versus longer increments . By studying the effect of frequency and peak strain on mechanically induced bone formation in the rat ulna loading model, Hsieh and Turner built a model that assumed that bone cells are activated by fluid shear stress and that the stiffness of the cells and the matrix around the cells increases at higher loading frequencies because of viscoelasticity. In this model, there is a strain threshold for an osteogenic response that varies with location. For example, in the proximal region of the ulna, the strain required to achieve new bone formation is 1300 µε, whereas different bone formation thresholds exist at the mid-shaft (2200 µε) and the distal region (3000 µε) . The major challenge has been to translate in vivo parameters of mechanical loading to in vitro cell culture models.
Even though osteocytes are thought to be mechanosensors , key questions such as how mechanical loading is sensed, how these signals are conveyed to other nonsensing cells and how these signals are translated into biochemical signals remain to be answered. Over the years, various theoretical and experimental studies argue that flow of interstitial fluid driven by extravascular pressure as well as by the applied cyclic mechanical loading is likely the means by which bone cells are informed of mechanical loading . It has been found that mechanical forces applied to bone cause fluid flow through the canaliculi surrounding the osteocyte that is probably responsible for the deformation of the cell membrane . Fluid flow imposes a shear stress on osteocytes, thus deforming the cells within their lacunae and the dendrites within their canaliculi. Perlecan (also known as HSPG2) in the pericellular matrix regulates solute transport and mechanosensing in osteocytes and mice lacking perlecan do not respond to loading . Theoretical modeling predicts osteocyte wall shear stresses resulting from peak physiologic loads in vivo in the range of 8–30 dyn/cm 2 . The first real-time attempts to measure solute transport in bone through dye diffusion within the lacunar-canalicular system have been conducted in vivo . These studies confirm earlier tracer studies of Beno et al. that the molecular weight cutoff for molecules in bone fluid is less than 7 nm, about the diameter of albumin. It is hoped that future studies will permit analysis of the effects of mechanical loading and blood pressure in this process ( Fig. 7.4 ).
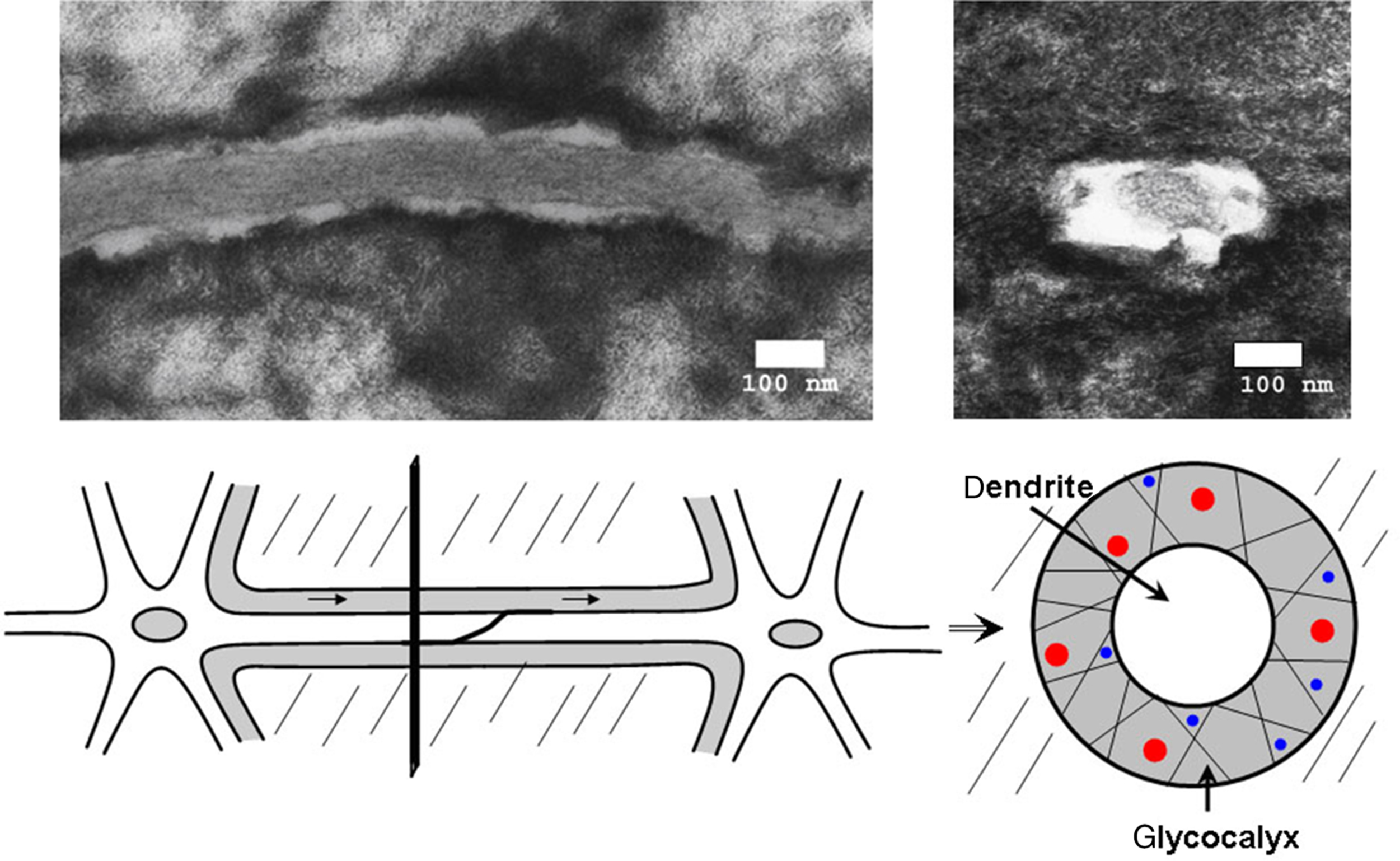
A model of strain amplification in osteocyte cell processes was proposed by Weinbaum et al. using a transmission electron microscopy–based model of the osteocyte process within its canaliculi at the osteocyte dendrite level. One of the requirements of the model is that osteocyte dendritic processes be tethered within canaliculi to the surrounding mineralized matrix through structural components, such as CD44, laminins, and a variety of other unknown proteins and proteoglycans present in the pericellular matrix surrounding the osteocyte. Another major requirement of the model is the formation of hexagonal actin bundles within the cell processes of the osteocyte. A relatively stiff structure can be generated with fimbrin predominately being cross-linked to actin bundles. The actin bundle is then attached to integrin related proteins through myosin type proteins, ERMs, and others. The model predicts that fluid flow through this structure will deform the shape of these tethering elements, creating a drag force predominately in this highly viscous, yet sieving pericellular matrix that then imposes a hoop strain on the central actin bundles in the osteocyte cell process. Wang et al. in 2007 expanded this model to include integrin attachment to conical projections on the canalicular wall that amplified strain to 10 times the hoop strain predicted by Han . These investigators have proposed that the osteocyte only senses mechanical load through its dendritic processes and that the osteocyte cell body is relatively insensitive to mechanical strain . Studies applying shear stress to either the cell body or the dendrites of MLO-Y4 cells suggest that the glycocalyx present on the surface of dendritic processes, but not the cell body, plays an essential role in mechanotransduction by dendrites but the cell body is still responsive.
It has also been proposed that mechanical information is relayed in part by cell deformation . Typical in vivo strains in humans are on the order of 1200 µε (principal compressive strain) to 1900 µε (maximum shear strain) . These strains were determined using strain gages that covered an area approximately 1.8 by 3.6 mm containing thousands of cells and are, therefore, averages of osteocyte strain. Variations resulting from microstructural features or discontinuities in the bone matrix will affect the local strain or deformation sensed by individual bone cells. Measured microstructural strains at or near osteocyte lacunae were found to be up to three times greater than the average strains measured with an external strain gage . If bone damage (microcracks) is present, the perilacunar strain magnification near a microcrack tip can be as high as 15 times in vivo–measured bone strain. Recently, real-time measurement of load-induced solute transport has been performed, and these studies suggested a peak shear stress of 5 Pa (30 dyn/cm 2 ) suggesting that higher strains can occur in vivo .
Nicolella et al. reported that the osteocyte lacuna acts as a strain concentrator that amplifies the macroscopic strain applied to the whole bone, and this amplification factor is a function of the local perilacunar bone tissue material properties . Using a microstructural finite element analysis model, they found that changes in the osteocyte cell body and cell process modulus had little effect on the maximum strain in the osteocyte, the average strain in the cell process, or on the maximum strain in the lacuna. However, changing the material properties of the perilacunar matrix had the greatest impact on the strain transmitted to the osteocyte, with the maximum osteocyte strain relating inversely to the perilacunar tissue modulus. Therefore any mechanism that changes the material properties of the perilacunar matrix (see later) will have consequences on mechanosensation by osteocytes.
An in vivo approach has been taken to tackle the question of what magnitude of strain an osteocyte perceives and how magnitude correlates with biological response . These investigators have determined magnitude of strain (the effector) with mapped gene expression (early biological response) with bone formation (end biological result). This information was used to generate a three dimensional model correlating magnitude of strain with magnitude and location of DMP1 and MEPE gene expression (as these are highly expressed in osteocytes) with resulting areas of new bone formation on the bone surface. The data to date show that osteocytes can respond as a population to increased strain, and that the response of each individual osteocyte also correlates with magnitude of strain in its local environment.
In in vitro cell culture, numerous investigators have used osteoblast cell lines under the assumption that osteocytes will respond in a similar manner; however, primary osteocytes have been shown to be more sensitive than primary osteoblasts in the release of PGE 2 following both hydrostatic compression and pulsatile fluid flow treatment, with pulsatile fluid flow being most effective . MLO-Y4 osteocyte–like cells are several orders of magnitude more sensitive to fluid flow shear stress than 2T3 osteoblast-like cells and MC3T3 osteoblast cells . Osteoblast-like cells are less responsive to oscillatory flow (applied fluid shear stresses of −20 to +20 dyn/cm 2 ) than pulsatile fluid flow (applied fluid shear stresses of 0–20 dyn/cm 2 ) and steady fluid flow (applied fluid shear stresses 20 dyn/cm 2 ) . Osteoblasts do respond to mechanical loading with an increase in mineralization as shown using MLO-A5 cells grown on a three dimensional scaffold . Correlation and validation of shear stress used in tissue culture with those in vivo remains to be performed.
It was hypothesized in 2003 that the bending of primary cilium of an osteocyte by extracellular fluid sends signals into cells through gap junctions . MLO-Y4 cells and MC3T3 cells express primary cilia , and removing the cilia reduced the amount of prostaglandin released by MLO-Y4 cells in response to fluid flow shear stress cells . These authors state that the mechanism used by primary cilia in bone cells is distinct from that of kidney cells as primary cilia in bone cells do not appear to mediate calcium flux in response to fluid flow . Primary cilia signal through cAMP and adenylyl cyclase 6 and can mediate signaling between osteocytes and mesenchymal stem cells . It is well known that polycystin 1 and 2 (PC 1,2), encoded by the genes Pkd1 and 2, are part of a mechanosensing complex in renal cells and also play a role in normal bone structure . Mice with impaired PC1 function develop osteopenia . Deletion of PKD1 in osteocytes using the 10 kb Dmp1-Cre results in reduced bone mass in the young skeleton, which subsequently recovers in the adult skeleton. However, despite recovery, the skeleton of adult mice remains less responsive to anabolic load . The effects of deleting PC1 are reversed by deleting Kif3a, a transport protein known to play a role in the function of cilia . It is not clear how a single cilium on an osteocyte cell body within a lacuna can mediate these effects. Schaffler et al. have found cilia only on osteocytes close to the periosteal surface and not deeper in mature bone . They propose that bone cell cilia are chemical rather than fluid flow sensors and their function may be more related to mineralization than to mechanosensing.
In addition to mechanical loading, both ultrasound and electromagnetic fields have been thought to affect bone cell function. Low intensity pulsed ultrasound is a form of mechanical energy used to accelerate fracture repair and distraction osteogenesis. Osteoblasts respond to ultrasound by increased expression of osteocalcin and insulin-like growth factor 1, while osteocytes do not . Conversely, substrate stretch and PTH increase Ca 2+ influx in osteocytes, not osteoblasts suggesting that the anabolic effects of ultrasound are through the osteoblast and that osteoblasts and osteocytes can respond distinctly to various forms of mechanical force. Pulsed electromagnetic fields increase TGFβ and PGE 2 in the osteocyte-like cell line, MLO-Y4, but decrease Cx43 expression in these cells as well as ROS17/2.8 osteoblast-like cells . As pulsed electromagnetic fields have been used to treat ununited fractures, these healing effects may be partially mediated by the induction of bone anabolic factors such as TGFβ and PGE 2 and by reducing osteocyte communication through Cx43 containing gap junctions. TGFβ produced by osteocytes could be delaying osteoblast differentiation, while increasing bone matrix volume .
Integrins have been proposed to play a role in mechanotransduction. Integrins, comprising heterodimers of α and β subunits, are major receptors/transducers that connect the cytoskeleton to the extracellular matrix and have been proposed to be candidate mechanosensors in bone cells . Stretch and fluid flow shear stress stimulate pathways that are regulated by integrin binding to the extracellular matrix . Among various isotypes of integrins, α5 and β1 integrins are expressed in virtually all cell types in bone . Integrins interact with plasma membrane proteins such as metalloproteases, receptors, transporters, and channels mainly through the extracellular domain of their α subunits . The evidence for the involvement of integrins in gap junction communication and Cx43 expression has been reported . The integrin alpha 5 appears to act as a tethering protein that responds to shear stress by opening hemichannels in osteocytes . Integrin α5β1 interacts with Cx43 independent of the integrin’s association with fibronectin and interaction with the extracellular matrix . Therefore fluid flow shear stress may have two major effects on the osteocyte mediated through integrins. The first is the well-known kinetics of the integrin acting as a linker between the extracellular matrix and the intracellular cytoskeleton. The second, novel effect is through the opening of hemichannels releasing small molecules such as prostaglandin with autocrine and paracrine effects. However, recently, another function for integrins αvβ5/3 on osteocytes has been identified, that of acting as a receptor for irisin to initiate the CREB signaling pathway to prevent apoptosis in osteocytes . This provides a third function for integrins in osteocytes.
7.8
Osteocyte signals for bone formation
Nitric oxide (NO) is a short-lived free radical important for the function of many tissues and organs. In bone, NO inhibits resorption and promotes bone formation. NO reduces osteocyte apoptosis . Both osteoblasts and osteocytes release NO in response to mechanical strain or fluid flow shear stress . NO can be generated from any of three isoforms of nitric oxide synthase, known as neural (n), endothelial (e), and inducible (i) NOS. Osteoblasts and osteocytes have highest expression of eNOS compared to the other synthases. eNOS-positive osteocytes in cases of femoral hip fracture are reduced in the inferior but not the superior region of the femoral neck compared to normal controls suggesting that eNOS-positive osteocytes act as sentinels to confine osteoclast activity to single osteons. Even though studies have shown little or no expression of iNOS in osteocytes, mice lacking this enzyme fail to regain bone after immobilization . These mice show no significant bone abnormalities unlike mice lacking eNOS in which bone growth is retarded. Surprisingly, iNOS has no effect on resorption in the unloading phase but is essential for bone formation in the reloading phase. iNOS expression was only found after unloading and reloading of bone, not in the normal loaded state.
ATP is released within seconds in osteoblasts in response to mechanotransduction and initiates intracellular calcium release. The P2X7 nucleotide receptor is an ATP-gated ion channel expressed in many cell types but appears to play a role in skeletal mechanotransduction . Deletion of this receptor results in mice with an attenuated inflammatory response and reduced bone formation . Macrophages from these animals do not release IL-1 in response to ATP. Skeletal sensitivity to mechanical loading was reduced about 70% in these null mice . Fluid flow shear stress did not induce prostaglandin release in cells isolated from these mice. Blockers of P2X7 receptors suppressed prostaglandin release, whereas agonists enhance release in MC3T3 osteoblast and MLO-Y4 osteocyte cells. The authors conclude that P2X7 receptor is necessary for release of prostaglandin in response to mechanical load.
Clearly, prostaglandin is a bone anabolic factor and osteocytes produce prostaglandin in response to load. Prostaglandins are generally thought to be skeletal anabolic agents as their administration can increase bone mass in humans and animals , stimulate bone formation in vitro in organ culture , and increase nodule formation in rat calvarial osteoblasts . Primary osteocytes and primary calvarial bone cells have been shown to release prostaglandins in response to fluid flow treatment . A number of studies have suggested that osteocytes are the primary source of these load-induced prostaglandins . In vivo studies have shown that new bone formation induced by loading can be blocked by the prostaglandin inhibitor, indomethacin , and that it is the inducible Cox-2 pathway that is primarily involved. Prostaglandin receptor agonists have been shown to increase new bone formation . However, others have found that Cox-2 null mice still respond to mechanotransduction . These authors suggested compensation through Cox-1 elevation.
It was hypothesized as early as 2002 that Lrp5 is a major player in the way that bone cells sense and respond to mechanical load . These investigators were responsible for the discovery of the high bone mass (HBM) gene, a mutation in the Lrp5 receptor (see also Chapter 18: Genetics of osteoporosis ). They reasoned that the HBM mutation results in a skeleton that is overadapted in relation to the actual loads being applied, but yet the skeleton is in homeostatic equilibrium. They found that wild-type bone experienced 40% greater strain than HBM bone with the same load. Based on these observations in humans and mice, they hypothesized that the set-point for load responsiveness was lower in the HBM skeleton. Loss of function mutations in Lrp5 results in low bone mass , but more importantly, the bones do not respond to mechanical load again supporting the notion that Lrp5 is involved in mechanosensation. It was the discovery that Lrp5 could regulate bone mass which brought attention to the wnt/β-catenin signaling pathway in the maintenance of bone mass.
Not only are positive regulators of bone formation produced by osteocytes but also negative regulators such as Dkk1 and sclerostin. These components of the wnt/β-catenin pathway in addition to Lrp5 have been shown to have major effects on bone mass. Although Dkk1 is expressed in many cell types, sclerostin is mainly expressed in osteocytes and articular hypertrophic chondrocytes . It has been proposed that the downregulation of Dkk1 and Sost create a permissive environment in which Wnt proteins already present can activate the Wnt pathway (for review see Ref. ). Targeted deletion of β-catenin in either osteoblasts or osteocytes has dramatic effects on bone. Deletion in osteocytes results in a bone phenotype of severe osteoporosis with a “moth-eaten” appearance . These investigators found that β-catenin is required for the expression of the antiosteoclastogenic factor osteoprotegerin, OPG, in osteocytes and that osteocytes express RANKL and OPG at levels exceeding those expressed by osteoblasts supporting the concept that osteocytes can recruit osteoclasts. β-catenin may also maintain bone mass by protecting osteocyte viability and preventing osteocyte apoptosis . Mechanical loading of MLO-Y4 cells by fluid flow shear stress protects against dexamethasone induced apoptosis through induction of PGE 2 crosstalk with the β-catenin signaling pathway . Both PGE 2 and fluid flow shear stress result in increased phosphorylation of GSK-3β and β-catenin nuclear translocation . In addition to playing a role in osteocyte viability in response to shear stress, the β-catenin pathway is important in osteocyte communication. β-catenin binds to the Cx43 promoter, stimulating Cx43 expression and functional gap junctions between osteocytes . The estrogen receptor alpha isoform (ER-α) may play a role in shuttling β-catenin into the nucleus in response to mechanical strain . This may in part explain how estrogen regulates bone mass through crosstalk between ER-α with Wnt/β–catenin signaling.
Estrogen has been proposed to modulate skeletal response to strain. Ehrlich et al. found that about 14% of all osteocytes were positive for estrogen receptor, ERα, under normal locomotion, but this number was decreased to 7.5% after a 2-week loading regimen that results in new bone formation in rat ulnae . The distribution of positive cells was uniform and did not correlate with peak strain magnitude suggesting that osteocytes respond to strain as a population. The response of mice deficient in ER-α and ER-β is inadequate to mechanical loading . It has been proposed that TGFβ III present in MLO-Y4 conditioned media enhances the production of estrogen which inhibits osteoclastic bone resorption . Conditioned media from osteocyte-like MLO-Y4 cells has also been shown to selectively stimulate the proliferation of mesenchymal stem cells and their differentiation into osteoblasts, but the factors responsible are not known . Estrogen has also been proposed to be an antiapoptotic factor for osteocytes (see the next section).
7.9
Osteocyte signals for bone resorption
Power et al. found elevated osteocyte density and lacunar occupancy in resorbing and forming osteons compared to quiescent osteons, leading to their conclusion that osteocytes may contribute to processes initiating or maintaining bone resorption . Isolated avian osteocytes have been shown to support osteoclast formation and activation . Like isolated chick osteocytes, the osteocyte-like cell line, MLO-Y4, was also found to support osteoclast formation, however unlike any previously reported stromal cell lines, did so in the absence of any osteotropic factors . These cells express RANK ligand along their dendritic processes and secrete large amounts of macrophage colony stimulating factor, both essential for osteoclast formation. Expression of RANK ligand along osteocyte dendritic processes provides a potential means for osteocytes within bone to interact and stimulate osteoclast precursors at the bone surface. MLO-Y4 cells and primary osteocytes can both support mesenchymal stem cell differentiation and osteoblast differentiation and also support osteoclast formation supporting the hypothesis that osteocytes have the capacity to regulate all phases of bone remodeling.
It was previously thought that osteoblasts were responsible for osteoclast formation and activation through the expression of RANKL. Even when RANKL expression was detected in osteocytes in vivo and MLO-Y4 cells in vitro , it was still not accepted that osteocytes could perform this function. The importance of osteocytes in regulating osteoclastic activity was recently confirmed in elegant in vivo studies performed by Xiong and O’Brien and Nakashima et al. , in which RANKL was deleted specifically in osteocytes. These mouse models developed an osteopetrotic phenotype, which led the authors to conclude that osteocyte-derived RANKL is essential for normal bone remodeling in adult mice. Osteocytes and MLO-Y4 cells are also known to express osteoprotegerin (OPG), a decoy receptor for RANKL . Deletion of β-catenin in osteocytes using Dmp1-Cre resulted in decreased expression of OPG and a subsequent increase in the RANKL/OPG ratio . These mice were characterized by severe osteoporosis, which was a consequence of enhanced osteoclast activity and excessive bone resorption. Therefore the expression of RANKL and OPG in osteocytes plays a role in regulation of resorption.
One of the major means by which osteocytes may support osteoclast activation and formation is through their death. Osteocyte apoptosis can occur at sites of microdamage, and it is proposed that dying osteocytes are targeted for removal by osteoclasts. Verborgt et al. mapped the expression of an antiapoptotic molecule called Bcl-2 and a proapoptotic molecule called Bax in osteocytes surrounding microcracks and found that Bax was elevated in osteocytes immediately at the microcrack locus, whereas Bcl-2 was expressed 1–2 mm from the microcrack. The authors propose that those osteocytes that do not undergo apoptosis are prevented from doing so by active protection mechanisms suggesting that damaged, yet still viable, osteocytes can send signals.
7.10
Osteocyte apoptosis and autophagy
It has been proposed that the purpose and function of osteocytes is to die, thereby releasing signals to stimulate remodeling and serving to target particular skeletal sites at selected time points for resorption . Osteocyte apoptosis can occur by aging, immobilization, microdamage, lack of estrogen, and elevated cytokines, such as TNFα, that occur after menopause and during treatment with glucocorticoids. Osteocyte cell death can occur in association with pathological conditions, such as osteoporosis and osteoarthritis, leading to increased skeletal fragility . Such fragility is considered to be due to loss of the ability to sense microdamage and signal to other bone cells for repair . Osteocyte apoptosis has been implicated in targeting the bone remodeling processes, since osteocyte apoptosis occurs in association with areas of microdamage and is followed by osteoclastic resorption in mechanically challenged bone . The apoptotic region around microcracks was found to be surrounded by surviving osteocytes expressing Bcl-2, whereas dying osteocytes appeared to be the target of resorbing osteoclasts .
In addition to microdamage, other skeletal insults cause osteocyte apoptosis. Oxygen deprivation has been shown to promote osteocyte apoptosis, especially as seen in immobilization. Hypoxia inducing factor alpha is elevated leading to apoptosis and the induction of the osteoclastogenic factor, VEGF and osteopontin, a mediator of environmental stress and a potential chemoattractant for osteoclasts . Withdrawal of estrogen results in osteocyte apoptosis as does glucocorticoid treatment . These observations are relevant to disease as cytokines such as TNFα and interleukin-1 (IL-1) have been reported to increase with estrogen deficiency . Apoptosis may also play an important role in the third most common cause of osteoporosis, glucocorticoid-induced osteoporosis .
Several agents found to reduce or inhibit osteoblast and osteocyte apoptosis include estrogen and selective estrogen receptor modulators , bisphosphonates and calcitonin , CD40 Ligand , and Calbindin-D28k . The pathways for some of these antiapoptosis agents have been extensively studied and dissected. For example, bisphosphonates appear to inhibit apoptosis through interaction with hemichannels and the ERK pathway , and Fas/CD95 plays a role in glucocorticoid-induced osteocyte apoptosis . Hence osteocyte viability may play a significant role in the maintenance of bone homeostasis and integrity. However, although blocking osteocyte apoptosis may improve conditions such as bone loss due to aging or to glucocorticoid therapy, osteocyte apoptosis may be essential for damage repair and normal skeletal replacement. Any agents that block this process may exacerbate conditions in which repair is required.
The osteocyte is generally a long-lived cell, surviving for decades within the bone microenvironment. Therefore this cell must possess mechanisms to maintain viability under conditions of stress. Xia et al. were the first to propose that osteocytes may undergo autophagy in response to stress such as glucocorticoid treatment . They showed that glucocorticoid was associated with the induction of markers and organelles associated with autophagy in osteocytes. Autophagy (“auto,” self; “phagy,” eat) is a mechanism by which the cell selectively degrades its own cytoplasm and selective organelles in an attempt to stay alive under conditions of stress. It is a tightly regulated process of lysosomal “self-degradation” in which the cell degrades and recycles nonessential cellular components to reuse them in processes necessary for survival. Autophagy can therefore protect cells from apoptosis, thereby preserving viability until the stress can be relieved. However, should the stress not be relieved, the outcome can be cell death. Other investigators have also shown glucocorticoid-induced osteocyte autophagy in vivo , where it was shown that low dose glucocorticoid-induced autophagy and high-dose glucocorticoid-induced apoptosis. Targeted deletion of the autophagy gene, ATG7, using the Dmp1-Cre driver, was shown to reduce autophagy in osteocytes, resulting in mice with low bone mass . These data suggest that the long-lived osteocyte requires autophagy to survive not only stressful conditions, but just to survive for decades within its enclosed environment.
7.11
Osteocyte modification of their microenvironment
Over 100 years ago, in 1910, Von Recklinghausen described enlarged lacunae in patients with rickets or osteomalacia and suggested that “pericellular digestion” was occurring . Over five decades ago, it was proposed that osteocytes may resorb their lacunar wall under certain conditions . The term “osteolytic osteolysis” was initially used to describe the enlarged lacunae in patients with hyperparathyroidism and later in immobilized rats . Bonucci and Gherardi suggested that poor mineralization at the time when the osteocyte is being embedded is the reason for enlarged lacunae with renal osteodystrophy. The term “osteocyte halos” was used by Heuck to describe pericanicular demineralization in rickets and later by others to describe periosteocytic lesions in X-linked hypophosphatemic rickets , a condition due to an inactivating mutation in Phex. Such periosteocytic lesions are not present in other chronic hypophosphatemic states. Recently it has been shown that Hyp mice have increased osteocyte expression of Ctsk, Trap, Mmp13, and Atp6v0d2 that could be partially rescued by treating the mice with either daily 1,25(OH) 2 D 3 or an FGF23 neutralizing antibody . “Perilacunar osteolysis” and “osteocytic osteolysis” was also described in animals such as rats sent into space for 22 days , in alveolar bone of hibernating ground squirrels , and in breeding female or hibernating snakes . Osteocyte removal of mineral from lacunae and canaliculi has important implications with regard to mineral homeostasis, changes in magnitude of fluid shear stress in bone, and changes in mechanical properties of bone.
In addition to hyperparathyroidism and hypophosphatemia, perilacunar remodeling has been observed in diseases including osteoporosis and glucocorticoid-induced osteoporosis . Glucocorticoids, in addition to having effects on apoptosis, may have direct effects on osteocytes resulting in modification of their microenvironment. It appears that glucocorticoid-treated subjects fracture at higher BMD values than postmenopausal women, but the reason is unclear . Mice injected with pellets releasing prednisolone showed an enlargement of osteocyte lacunae in trabecular bone and the generation of a surrounding sphere of hypomineralized bone . Lacunae act as stress concentrators in bone; therefore it was proposed that these highly localized changes in bone properties may influence fracture risk in glucocorticoid-treated patients . It was suggested that glucocorticoids may alter or compromise the metabolism and function of the osteocyte, not just induce cell death.
After the 1960s and 1970s the concept of “osteocytic osteolysis” began to decline as did the number of publications on the topic (for a review see Ref. ). One strong argument against “osteolytic osteolysis” was the observation that enlarged lacunae could be found around younger osteocytes due to defective mineralization while embedding . Another reason that the concept fell out of favor is because “osteocytic osteolysis” has frequently been confused with the resorption mechanisms used by osteoclasts. When primary avian osteocytes were seeded onto dentin slices, no resorption was detected; therefore these investigators concluded that osteocytes cannot remove mineralized matrix . However, one must keep in mind that removal of mineral by osteocytes (weeks/months) would certainly be slower than osteoclastic resorption (days) and therefore not detectable using this approach. It must also be kept in mind that the osteocyte cannot form a resorption lacuna complete with sealing zone, but it does reside within a lacuna.
More than three decades ago, it was suggested that the osteocyte not only has the capacity to destroy matrix but also to form matrix and that the osteocyte can remodel its local environment including lacunae and canaliculi . Osteocyte lacunae were shown to uptake tetracycline, called “periosteocytic perilacunar tetracycline labeling” indicating the ability to calcify or form bone. Greater solubility of the intralacunar mineral surrounding the normal osteocyte was also found . Interestingly, Baylink and Wergedal had described TRAP activity in osteocytes in 1969 , a finding that was criticized as potentially being due to a diffusion artifact from osteoclasts. However, this observation was validated by Nakano et al. using in situ hybridization for TRAP gene expression . Baylink and Wergedal showed tetracycline binding to the perilacunar matrix, suggesting that osteocytes have the ability to replace their perilacunar matrix . In 1983 Zallone et al. also reported tetracycline labeling in osteocyte lacunae in egg-laying hens . These observations suggest that the osteocyte can both add and remove mineral from its lacunae and canaliculi.
Osteocyte resorption and replacement of their perilacunar matrix became more accepted after investigators could reproduce the studies of Qing et al. . They showed that osteocyte lacunar area increases with lactation and returns to normal with forced weaning. Genes thought to be osteoclast specific such as TRAP and Cathepsin K were found to be elevated in osteocytes during lactation and returned to normal with weaning. PTHrP, which is known to be elevated in the circulation during lactation, was found to reproduce these effects on lacunar enlargement and confirmed to be mediated through the PTH type 1 receptor. This study shows that healthy osteocytes can both remove and replace their perilacunar matrix during normal reproductive function, suggesting that osteocytes play an important role in mineral homeostasis during a calcium-demanding condition such as lactation. It was suggested that “osteocytic osteolysis” be reserved for pathological conditions, whereas the term “perilacunar remodeling” be used for the function of the healthy osteocyte such as in the lactating animal. It has recently been shown that the increases in lacunar area but not material properties with lactation alter the mechanical properties of bone .
Recently, the molecular mechanisms responsible for perilacunar remodeling have been identified. Lactation and PTHrP were shown to decrease osteocyte extracellular pH in vivo and in vitro . Osteocytes are resistant to mild acidification compared to osteoblastic cells, suggesting that they can remain viable during removal of their perilacunar matrix. Lactating mice with a global deletion of the calcitonin receptor showed an increase in lacunar area compared to lactating wild-type mice . Sclerostin appears to increase perilacunar remodeling genes and increase osteocyte lacunar area . Administration of recombinant sclerostin to MLO-Y4 cells and primary human osteocytes was shown to induce the expression of perilacunar remodeling genes such as Ctsk, Car2, and Mmp13 .
In summary an old observation by early “pioneers” has now been validated with modern research approaches. It is estimated that there are up to 42,000,000,000 osteocytes in the adult human skeleton, with a total surface area of 215 m 2 in the lacunocanalicular system . The ability of osteocytes to release even a small amount of calcium from their perilacunar matrix would have a substantial effect on the overall serum calcium level. Release of calcium by osteocytes may limit the need for osteoclastic bone resorption, protecting bone mass and structure.
7.12
Osteocyte regulation of phosphate metabolism
Osteocytes appear to regulate phosphate and biomineralization through molecules such as PHEX, DMP1, and MEPE , all highly expressed in osteocytes . These three molecules have well-characterized effects on the skeletal system, as demonstrated by the loss of function mutations and knock-out mouse models . Dmp1-null mice have a similar phenotype to hypophosphatemic (Hyp) mice carrying a Phex mutation, that of osteomalacia and rickets due to elevated FGF23 levels in osteocytes , whereas the MEPE nulls have increased bone mass . The human condition, autosomal dominant hypophosphatemic rickets, is due to mutations in PHEX and autosomal recessive hypophosphatemic rickets is due to mutations in DMP1 . In the absence of either DMP1 or PHEX, FGF23 is dramatically elevated in the osteocyte and in the circulation, leading to phosphate excretion by the kidney, thereby reducing circulating phosphate levels and resulting in osteomalacia and rickets. FGF23 is not normally expressed at high levels in osteocytes but is dramatically upregulated in both DMP1- and PHEX-associated hypophosphatemic rickets and in chronic kidney disease, CKD . Based on these observations, it was proposed that the osteocyte lacunocanalicular network can function as an endocrine system, targeting distant organs such as kidney .
The exact mechanism(s) by which DMP1 and PHEX regulate FGF23 are yet to be determined; however, recent research has suggested that their inhibitory effects are mediated by FGF receptor (FGFR) signaling . DMP1-null and Hyp mouse models show enhanced FGFR signaling compared to wild-type control mice and inhibition of FGR signaling resulting in reduction of FGF23 in the bone marrow stromal cells from both mouse models.
The effects of MEPE on FGF23 and matrix mineralization are variable and dependent on release of an acidic serine aspartate-rich MEPE-associated motif (ASARM) from its C-terminus. This 19 amino acid ASARM peptide is known to be a potent inhibitor of mineralization in vivo and absence of this peptide in MEPE-null mice may be the reason these mice have increased bone mass and mineral apposition rate with aging. MEPE and the MEPE-ASARM peptide can have different effects. Binding of MEPE to PHEX prevents its proteolytic degradation and the release of the ASARM peptide , preventing downregulation of FGF23. The ASARM peptide is known to bind specifically to PHEX in vitro to inhibit PHEX enzymatic activity , which results in upregulation of FGF23 expression. In addition, the phosphorylated MEPE-ASARM peptide itself is a substrate for PHEX, with cleavage of ASARM by PHEX neutralizing its activity and restoring mineralization . This is a very complex regulatory system and requires further study.
Since its identification in 2000 in the ventrolateral thalamic nucleus of the brain , FGF23 has actually been found to be most highly expressed in bone, predominantly in the osteocyte . In addition to being regulated by Dmp1, PHEX, and MEPE, 1,25(OH) 2 D induces the expression of FGF23 in the osteocyte as shown in murine and cell culture models , suggesting a negative feedback system. In addition to 1,25(OH) 2 D, PTH may directly regulate FGF23 levels. Infusion of PTH in mice resulted in increased FGF23 mRNA expression in the calvaria and increased serum FGF23 . PTH was also shown to upregulate expression of FGF23 mRNA in UMR106 cells. Rhee et al. observed an increase in FGF23 expression in osteocytes in transgenic mice with constitutive activation of the PTH receptor (PTHR1) in osteocytes under control of the DMP1 promoter . The consequences and implications of elevated FGF23 in disease will be covered later.
7.13
Osteocyte communication with muscle
Dogma has been that mechanical loading of bone through muscle contraction is the sole or main interaction between muscle and bone. However, new data suggests that the bone and muscle may communicate through soluble mediators. In 2015 and 2016 it was shown that osteocalcin can restore muscle mass . Recently it has been shown that osteocytes secrete factors that regulate muscle mass and function. MLO-Y4 osteocyte-like cells and primary osteocytes secrete factors that support muscle myogenesis and activation of the Wnt/β-catenin pathway in C2C12 cells . Two factors produced by osteocytes in response to shear stress, PGE 2 and Wnt3a, were found to enhance myogenesis and ex vivo primary muscle function. Therefore several lines of in vitro and in vivo evidence are emerging to support that hypothesis that bone cells can regulate muscle mass and function through secreted factors.
Not only do osteocytes in bone function as an endocrine organ, but so does muscle . In 2007 Pedersen coined the term “myokines,” for muscle-secreted factors . Myokines include inhibitors such as myostatin, a potent inhibitor of skeletal muscle cell proliferation and growth and stimulators such as IL-8, brain-derived neutrophic factor, IL-15, a muscle factor that reduces adiposity, and irisin a potent regulator of the conversion of white fat into brown fat (for review see Ref. ). Secreted factors from C2C12 myotubes but not from C2C12 myoblasts were shown to increase the viability of MLO-Y4 osteocyte-like cells treated with dexamethasone, and intact skeletal muscles electrically stimulated ex vivo also protect osteocytes against cell death .
Irisin, the shed extracellular domain of a transmembrane protein called FNDC5, is produced mainly in response to physical activity and contraction and is a central regulator of muscle and fat metabolism . It is a promyogenic factor capable of enhancing skeletal muscle size in experimental models of immobilization- and denervation-induced atrophy. Irisin was also shown to increase cortical bone mass . Although several studies have shown that very low dose irisin will support bone formation, a more recent study shows that global deletion of FNDC5 results in total protection from bone loss due to ovariectomy . These apparently contradictory results remain to be resolved .
β-Aminoisobutyric acid, BAIBA (103.6 Da), is secreted by contracting muscle to target other tissues, including bone . This is a small metabolite produced from valine though the actions of PGC1α. The metabolite has been shown to influence a number of metabolic processes such as increasing energy expenditure by activating the β-oxidation pathway of hepatic fatty acid, triggering the browning of white adipose tissue, improving insulin resistance and inflammation in skeletal muscle in autocrine/paracrine manner, reducing hepatic ER stress and glucose/lipid metabolic disturbance in type 2 diabetes, and reducing renal fibrosis in mouse models of obstructed kidney via inhibition of renal fibroblast activation and fibrosis . Levels of this metabolite are inversely correlated with cardiometabolic risks factors . Recently, it has been shown that this metabolite prevents the loss of bone with hindlimb unloading apparently through functioning as an osteocyte protective factor against reactive oxygen species .
7.14
Role of gap junctions and hemichannels in osteocyte communication
Clearly, osteocytes can communicate extracellularly through the production of small molecules such as NO, ATP, prostaglandins, and secretion of larger proteins such as FGF23 and Sclerostin. Turner et al. have suggested that bone cells may communicate in a fashion similar to neural cells through molecules such as glutamate, serotonin, leptin, and neuropeptide Y2 that are responsible for habituation, sensitization, and long-term memory. Osteocytes do not express functional glutamate receptor but do express GLAST, a molecule that sequesters glutamate, suggesting that the osteocyte may signal to responding osteoblasts and osteoclasts that do express the receptor . Serotonin receptors have also been found on osteocytes, the 5-HT(2B) receptor is higher on avian osteocytes than osteoblasts and serotonin has been shown to increase bone mineral density . Though intriguing to view bone as a neuronal network, further studies are required.
Another means by which osteocytes communicate is intracellularly through gap junctions. The cell processes of osteocytes are connected with each other and with cells on the bone surface via gap junctions , thereby allowing direct cell-to-cell coupling. Gap junctions are transmembrane channels, which connect the cytoplasm of two adjacent cells. These channels permit molecules with molecular weights less than 1 kDa to pass through and have been shown to modulate cell signaling and tissue function in many organs and cells. Gap junction channels are formed by members of a family of proteins known as connexins. Functional gap junctions in osteoblasts were first identified by injection of fluorescent dye into rat calvarial subperiosteal osteoblasts that spread to neighboring osteoblastic cells . Gap junctions and Cx43 are important for osteoblast differentiation, and the functions and expression of gap junctions and Cx43 are regulated by prostaglandins, hormones, and other signaling molecules. Cx43-null mice have delayed ossification, craniofacial abnormalities, and osteoblast dysfunction .
It has been proposed that gap junctions function through the propagation of intracellular signals contributing to mechanotransduction in bone, thereby regulating bone cell differentiation . A dominant negative mutant of Cx43 diminishes fluid flow–induced release of PGE 2 , but not Ca 2+ responses . In addition, the fluid flow–induced PGE 2 response of osteoblastic ROS17/2.8 cells is gap junction–mediated and independent of intracellular Ca 2+ . Fluid flow–induced shear stress stimulates gap junction–mediated intercellular communication and increases Cx43 expression in osteocyte-like MLO-Y4 cells . PGE 2 released in response to fluid flow functions in an autocrine fashion to activate EP 2 receptor signaling, including increased intracellular cAMP and activated PKA, which in turn, stimulates gap junction function and Cx43 expression . Oscillating fluid flow has been shown to upregulate gap junction communication in MLO-Y4 cells by an ERK1/2 MAP kinase-dependent mechanism . Yellowley et al. have shown that the osteocyte-like MLO-Y4 cells can couple through gap junctions to osteoblast-like MC3T3 cells .
Hemichannels were identified in osteocytes in addition to other potential openings or channels to the extracellular bone fluid such as calcium-, ion-, voltage-, and stretch-activated channels . Osteocytes and MLO-Y4 osteocyte-like cells express large amounts of Cx43, the component of gap junctions, but these cells are only in contact through the tips of their dendritic processes. This raised the question of how Cx43 located in the rest of the cell membrane could be functioning. Connexins can form and function as unapposed halves of gap junction channels called hemichannels, localized at the cell surface, independent of physical contact with adjacent cells . Functional hemichannels formed by Cx43 has been reported in neural progenitor cells and neurons, astrocytes, heart, and osteoblasts and osteocytes. The opening of hemichannels appears to provide a mechanism for ATP and NAD + release, which raises intracellular Ca 2+ levels and promotes Ca 2+ wave propagation in astrocytes, bone cells, epithelial cells, and outer retina. Hemichannels expressed in bone cells such as MLO-Y4 cells appear to function as essential transducers of the antiapoptotic effects of bisphosphonates . Hemichannels formed by Cx43 directly serve as the pathway for the exit of elevated intracellular PGE 2 in osteocytes induced by fluid flow shear stress . This is the first report of modulation of hemichannel function in response to mechanical stress. Hemichannels forming gap junctions at the connecting tips provide intracellular communication and hemichannels along the dendrite are the conduit for extracellular communication. As stated earlier, integrin α5β1 interacts with Cx43 to mediate the opening of hemichannels in response to mechanical stimulation, which is responsible for the regulation and extracellular release of prostaglandin. A focus has been on the role of Cx43 on prostaglandin release, but clearly gap junctions and hemichannels are intercellular and extracellular portals for other unknown signaling molecules.
7.15
Osteocytes in the embryonic and the adult skeleton with aging
Mechanical strain is absolutely required for postnatal, but less for prenatal skeletal development and maintenance as excised embryonic metatarsals will continue to develop ex vivo. Mice lacking Dmp1, Phex, MEPE, Sost, and other proteins that are highly expressed in osteocytes do not show a phenotype until days to weeks or even months after birth . One potential explanation for this is that functional osteocytes are not required in the embryo. Osteocytes may act as “place holders” in the embryo until they can assume their functions as mechanosensors in the postnatal or adult skeleton. Also, in utero, although subjected to some mechanical loading via muscle insertions, the skeleton is not subjected to significant loading from weight bearing activity. Therefore responses of load-related bone remodeling are less significant in the developing embryo. Growth and development are the overriding signals prenatally compared to any loading or unloading signals. Their extensive dendrite connections also may not be required because the bone cortices and trabeculae are relatively thin and poorly mineralized and the cells are near the surface . Thus nutrients may be able to readily diffuse to the osteocytes without requiring an extensive canalicular system. Therefore molecules that play a role in the responses of osteocytes to mechanical strain may not reveal their importance for normal skeletal physiology until postnatally or in the adult animal. Osteocyte biology and function may be more relevant to adult disease than to development.
It is not clear if a relationship exists between osteocyte density and bone volume and remodeling. Jordan et al. hypothesized that in the cases of osteoarthritis, increased TGFβ may decrease the conversion of osteoblasts to osteocytes, thereby decreasing osteocyte density and increasing bone mass based on studies showing that the inactivation of the TGFβ pathway led to the opposite effects . They examined patients with coxarthrosis known to have elevated TGFβ and found a reduction in osteocyte lacunar density and an increase in wall width in femoral neck biopsies consistent with their prediction. These observations support those of Karsdal et al. showing that osteoblast lifespan and matrix production before incorporation into matrix as an osteocyte appears to be regulated by TGFβ. In contrast, Vashishth et al. found that increasing osteocyte density was associated with increases in bone volume and that osteocyte lacunar density predicts cancellous and cortical bone volume . Qiu et al. found a correlation of increased osteocyte density with less bone remodeling . They found that osteocyte density declines with age but not with menopause, in deep bone but not superficial bone, and suggest that it is the age of the bone and not the age of the subject that determines osteocyte density. They propose that one function of remodeling is to maintain osteocyte viability. They also found that fracture patients had fewer osteocytes before fracture than healthy controls and conclude that osteocyte deficiency may contribute to bone fragility by impairing osteocyte detection of microdamage or by a reduction in canalicular fluid flow. These authors also found that black women have higher osteocyte density than white women , perhaps playing a role in increased bone strength. In black women as in white women, more empty lacunae were found in deep than in superficial bone and there was age-related loss of osteocytes.
Robling and Turner did not find a correlation between osteocyte density and mechanosensitivity in three strains of mice. They suggest that genetic components other than osteocyte density regulate mechanosensitivity . Clearly, further study is required to clarify the importance of osteocyte density in osteocyte function in bone.
The osteocyte, its network, and connectivity appear affected by age (for review see Ref. ). Multiple studies have shown that osteocyte lacunar density decreases with age which may be due to micropetrosis, the infilling of the lacunae with mineral . These observations in humans have now been reproduced in rodent models. Osteocyte density was found to be lower in the femurs of 22-month-old mice compared to 5-month-old mice, and a reduction in dendricity preceded a reduction in osteocyte number suggesting dendrites are necessary for viability .
As the osteocyte is the longest lived bone cell, it could be thought of as a memory cell that has a greater potential to undergo cellular senescence with aging compared to other short-lived cells. With aging, senescent osteocytes and myeloid cells predominantly express the senescence-associated secretory phenotype, SASP, compared to other lineages . Treating a mouse model that expresses the INK-ATTAC “suicide” transgene that deletes senescent cells when given a compound called AP20187 resulted in the prevention of bone loss . The mice were given the compound at 20 months of age for 4 months and not only were osteocytes targeted but so too were osteoclasts in this model. For review, see Pignolo 2019 .
7.16
The implications of osteocyte biology for bone disease
Osteocyte connectivity and lacunocanalicular organization may play a role in bone disease (for a review see Ref. ). The early formation of dendrites by embedding osteoid–osteocytes is polarized toward the mineralization front to which cellular processes are oriented. Cellular processes oriented toward blood vessels only begin to appear when the mineralization begins to spread around the cell . Osteocyte dendricity changes depending on orientation and with static and dynamic bone formation . In undiseased bone, osteocyte connectivity is high and the processes are oriented in the direction of the blood supply . In osteoporotic bone, there is a marked decrease in connectivity, as well as disorientation of the dendrites, which increases with severity . In contrast, in osteoarthritic bone, a decrease in connectivity is observed, but the orientation is intact. In osteomalacic bone the osteocytes appear viable with high connectivity, but the processes are distorted and the network chaotic . Changes in osteocyte dendricity could not only have a dramatic effect on osteocyte function and viability but also on the mechanical properties of bone. An equilibrium must be met between the number and branching of dendrites to preserve function and viability versus the number that would decrease bone strength ( Fig. 7.5 ).