Table 43.2
Pathogenesis of congenital angio-osteohypotrophy
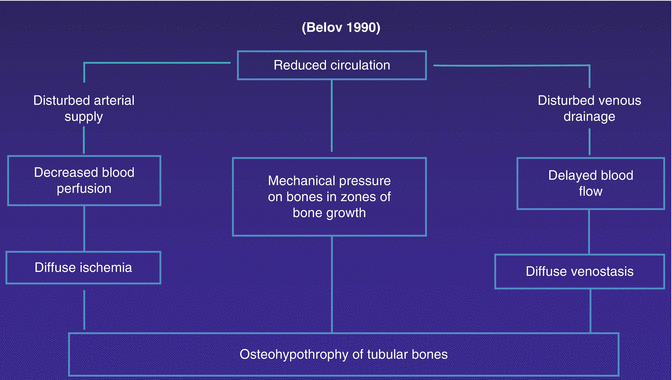
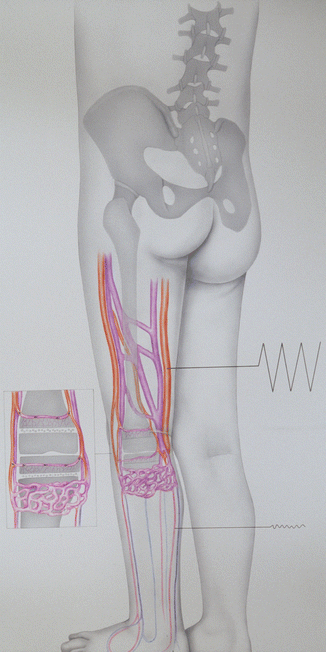
Fig. 43.1
Drawing explaining pathogenesis of congenital circulatory-hypoxic hyperostosis at the zones of bone growth in the region of the distal thigh and the proximal shank: large blood perfusion in the arteries of the thigh and hypotonia in the arteries of the shank. AV fistulas in the proximal shank lead to hypoxia of osteoblasts in bone metaphyses which signifies a stimulation to their activity resulting in hyperostosis of the tubular bone [35, 36]
The management of the length discrepancy requires an early removal of the hemodynamic and metabolic causes for the bone pathology because the likelihood of correction by this vascular operation to the primary lesion is much higher when done during infancy (Table 43.3) [12, 34, 35]. When, however, the long-bone length discrepancy is more than 4–5 cm, the combined approach of an orthopedic procedure and vascular surgery has been generally accepted as the primary strategy. When presented with severe length discrepancy, noninvasive and/or invasive diagnosis procedures should be started even before the age of 3 in order to perform the necessary vascular procedure to correct the pathologic hemodynamics first before it is too late [17]. However, if the vascular cause of the length discrepancy can be contained until the age of 7, orthopedic surgery may then be performed (Table 43.4). The policy of first performing vascular surgery to arrest further abnormal long-bone growth and then subsequent additional orthopedic correction is the treatment of choice. An early aggressive approach is mandatory [9, 30–32].
Table 43.3
Therapeutic strategies in congenital vascular defects
To start in early childhood (at the age of 3–7) |
To influence the pathophysiologic process and to abolish the hemodynamic dysfunction |
To perform a harmonized individual treatment |
To perform surgery radically without loss of function |
To perform a stepwise surgical treatment |
To practice a multidisciplinary therapy |
Table 43.4
Indications for an additional orthopedic treatment to correct a length discrepancy of the legs in angiodysplasias
If in a distinct length discrepancy (even after vascular surgery) the correction is not satisfying. |
After the end of growth (temporary epiphysiodesis being excluded). |
If there is no indication for vascular surgery to correct the length discrepancy. |
In the long run, length discrepancy may not represent the underlying problem in these patients. That is why a critical indication should be considered.
Children and young people with a congenital vascular malformation sometimes also suffer mono- or oligoarthralgia of the large joints, mainly of the lower limbs (Table 43.5) (Hauert Disease) [33].
Table 43.5
Distribution patterns of angiodysplasia arthropathy
N = 53 | ♂ | ♀ |
|---|---|---|
Knee | 10 | 26 |
Hips | – | 4 |
Ankle joint | 1 | 2 |
What we notice here is that this includes cases in which destruction of the affected joints occurs early on in childhood, sometimes even before the age of 5, the considerable extent of which stands in extraordinary contrast to the usual complaint of moderate or slight pain. What is also striking is that this joint condition is accompanied only by very marginal inflammatory malformations and that, entirely unlike in primary degenerative joint changes, this type of destruction appears not to show any significant tendency of progression after longitudinal growth is complete. There are no signs of systemic inflammation; rheumatoid factors are consistently negative [5–8]. The vertebra joints appear to be recessed. Based on our experience and knowledge of international literature, this very unusual clinical phenotype is as yet undocumented, so we have provisionally opted for the descriptive term of angiodysplasia arthropathy, or “Hauert disease,” for this set of conditions, with the aim of having it acknowledged as a stand-alone entity.
Vascular malformation on the affected limb makes most patients seriously ill in any case, a major symptom being chronic bouts of phlebitis causing pain and restriction of movement. The clinically visible signs of vascular malformation explain why it is only in later examinations that joint disease is considered as a cause of the symptoms. The vascular malformation alone is capable of compromising joint function so much so that its typical symptoms such as limping, contractures, and painful movement can obscure an arthropathy, which shares the same symptoms. The most striking symptom in patients is a loss of function of the joint, a limp, and possibly a periarticular swelling. Effusion in the knee joint or hemarthrosis is key exceptions. There are generally no lab-based inflammation parameters.
A restriction in movement of the ankle and foot joints can already be detected in newborns. Clinical examination using duplex ultrasound (color-coded ultrasound of the vessels) can quickly diagnose vessel failure (vascular malformation).
We have divided the disease into three degrees of severity depending on the extent of the tissue malformations (Table 43.6).
Table 43.6
Diagnostic and therapeutic algorithm
N = 53 | Severity level I | Severity level II | Severity level III |
|---|---|---|---|
Quantity | 11 | 24 | 18 |
Substrate | Synovial fluid | Synovial fluid, cartilage | Synovial fluid, cartilage, bone |
Plain X-ray | Unobtrusive, phlebolith | Irregularity of the subchondral plate, demineralization (in a comparison test) | Subchondral sclerosis, mutilations |
MRT | Thickening of the synovial fluid + vascular malformation | Cartilage reduction, vascular malformation | Destroyed bone, vascular malformation |
Symptoms | Limping, soft tissue inflammation (pain) | Reduced function, pain | Severe pain, contractures |
Treatment | Conventional, diagnostic arthroscopy, partial synovial fluid resection | Debridement | Debridement, Quengel orthoses, joint replacement |
Diagnostics
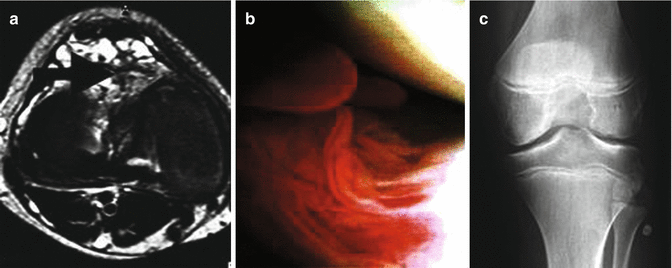
A plain radiological image in the early stages I and II shows almost no malformation. The earliest signs are irregularities in the subchondral plate (in a test comparison). A demineralization of the joint-adjacent bone may be detected – only rarely do we find signs similar to actual arthritic signs [2]. This recessed reaction in the bone may have contributed to the failure to diagnose during phlebographic examinations [20]. Subchondral sclerosis can occur from level II upwards which at level III leads to unmistakable malformations in the bones of the joint. Actual arthritic malformations with osteophytes etcetera are rare.
At the hip joint, the damage is localized mostly at the head with an extensive recess of the acetabular partner. We have never seen this develop into an ankylosis; the clinically detected movement restrictions were ligamentous and algogenic in nature. Phleboliths (calcifications in the malformed blood vessels) are a strong indicator of “Hauert disease” (HD).
MRT
MRT imaging presents the damage to the capsule, cartilage, and bone. At the same time, you can see the existing pathological vessels in the surrounding tissue (resolution up to 1 mm). Predominantly long, slow-flow vascular proliferations point to suspected vascular malformation. The sequences best suited to this are proton heavy with spectral fat saturation or, if not available, then turbo inversion recovery sequences. The proliferating giant-cell synovioma can be easily defined by proving blood degradation products such as hemosiderin of the venous malformations. The appearance of narrow-diameter venous malformations, partial thrombosis, or thromboses as well as by inflammatory changes in the walls requires the intravenous administration of contrast agents.
Arthroscopic Diagnosis
With level I malformations (Fig. 43.2), arthroscopy (Fig. 43.3) shows a highly irritated mucosa, part of which are the menisci. Vascular malformations can show through the synovial fluid membrane. Rarely do you also find individual malformations as soft, vascularized tumors in the joint. Yellow/brown plaque points to hemosiderin deposits which must then be distinguished from a giant-cell tumor of the tendon sheath. The examination is often complicated by pronounced forming of adhesion. With level II malformations (Fig. 43.4), you can recognize the random cartilage destruction and the oversized menisci. With level III malformations, the exposed bone is seen with red spots. You can see the white of the bone showing through where it is almost entirely stripped. These malformations are independent of the stress zones. Overall, the experienced arthroscopist will conclude an “unseen image.”