Fig. 33.1
Yakes Type I AVM (AVF) typified by a single inflow artery connected to a single outflow vein. (a) Ventilator Dependent 30 Year-Old Female with HHT and Massive Left Pulmonary AVM Causing O2 Sats of 35% on 100% Oxygen Through the Ventilator; Patient Sent By Air Ambulance Emergently For Treatment. Left Pulmonary Artery angiogram demonstrating a massive AVF shunt with single aneurysmal vein drainage. This single arterio-venous connection is Yakes Type I AVM (AVF). (b) Post-embolization selective Left Pulmonary Artery angiogram after placement of 22 fibered coils of .038 & .035 sizes in the AVF totally occluding the massive AVF. (c) Main Left Pulmonary Artery angiogram demonstrating closure of the massive AVF post-coil placement. Mechanical closure devices will permanently close and treat this Yakes Type I AVM (AVF)
The Yakes Type II Classification possesses an angioarchitecture synonymous with the classical “nidus” pattern commonly seen in AVMs with multiple inflow arteries of varying sizes coursing toward a “nidus” (a complex tangle of vascular structures without any intervening capillaries and exiting from this “nidus” into multiple veins from this “nidus”). The Houdart Type C and the Cho-Do Type IIIa/Type IIIb most resemble this angioarchitecture pattern. Thus, the Yakes Type II and Yakes Type IV further define the Houdart Type C and Cho-Do Type IIIa/IIIb patterns (Fig. 33.2), much more specifically.
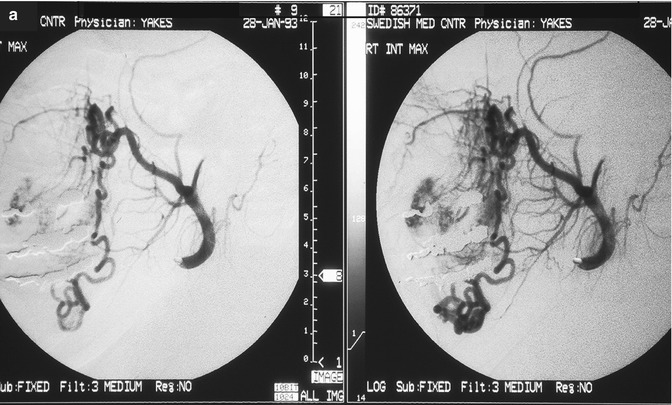
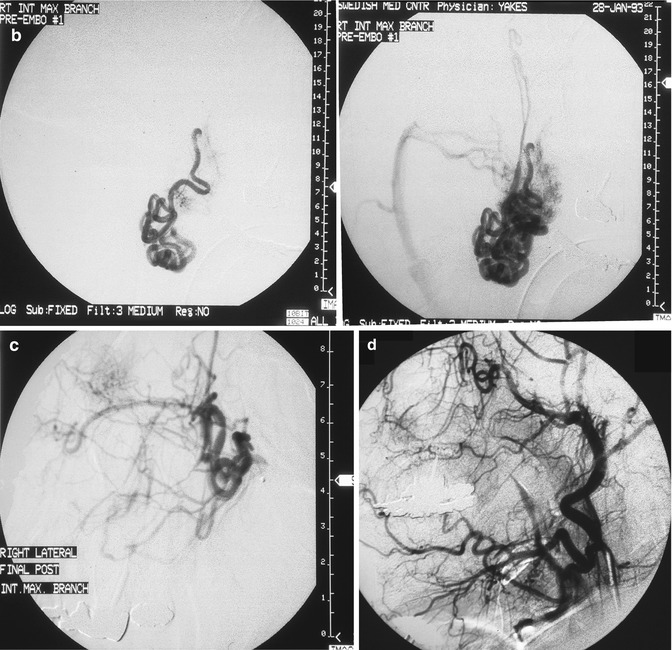


Fig. 33.2
24 year old female with painful right facial AVM also causing right facial swelling. (a) Example of Yakes Type II AVM with typical AVM “nidus”. This type AVM can be treated by trans-arterial embolization (easiest approach usually), and direct puncture into the nidus (more difficult). Retrograde vein approaches are usually not successful. Lateral Right Internal Maxillary Artery arteriogram demonstrating arterial supply from a terminal Internal Maxillary artery branch arising from the Pterygo-Palatine fossa area. Note the typical AVM “nidus pattern” (b) Lateral selective Right Internal Maxillary Artery branch arteriogram pre-embolization. A micro-catheter is required to obtain superselective arterial positioning for ethanol embolization of the AVM. This is required to ONLY embolize the AVM and spare all the normal tissues and capillary beds from ethanol arterial embolization. If not done this way, there will be total tissue devitalization and necrosis that will occur with inadvertant embolization of ethanol of the normal tissues. (c) Lateral Right Internal Maxillary Artery arteriogram immediately post-embolization demonstrating total occlusion of the right face AVM with all normal branches remaining intact. (d) Lateral Right External Carotid Artery arteriogram at 2 year follow-up. No residual AVM is identified. Note that the normal arterial vascularity remains intact
As an aside, the term “nidus” is rampant in the medical literature (AVM nidus, nidus of infection, etc.). Unfortunately, the initial author was only partially familiar with the Latin language. “Nidus” means “nest” in Latin, and indeed it does. However, “nidus” with the ending “us” denotes male gender. In the Latin language, the true term meaning “nest” is, in fact, “nidum.” The ending “um” denotes the neuter gender which a “nest” truly is. Thus, the original author accurately describing “nest-like” conglomeration of vascular structure was woefully inaccurate penning the words as “nidus” (masculine) instead of the true word “nidum” (neuter). Being rife in the literature for decades, there is no possibility of any correction of this term.
In summary, Yakes Type I is the simplest macro direct AV connection. Yakes Type II is the common “nidum” (nest-like) AV connection. Yakes Type IIIa has multiple AV connections (arterial and arteriolar into an aneurysmal vein: “nidum” is in the vein wall) with single outflow vein physiology (Fig. 33.3). Yakes Type IIIb has multiple arterial inflow connections (arterial and arteriolar) into an aneurysmal vein (“nidum” is in the vein wall) with multiple outflow veins that is more difficult to treat by retrograde vein approaches (Fig. 33.4).
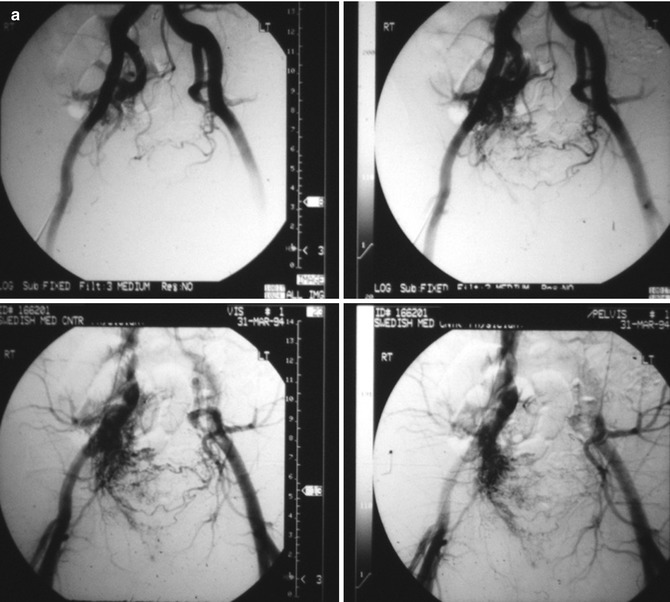
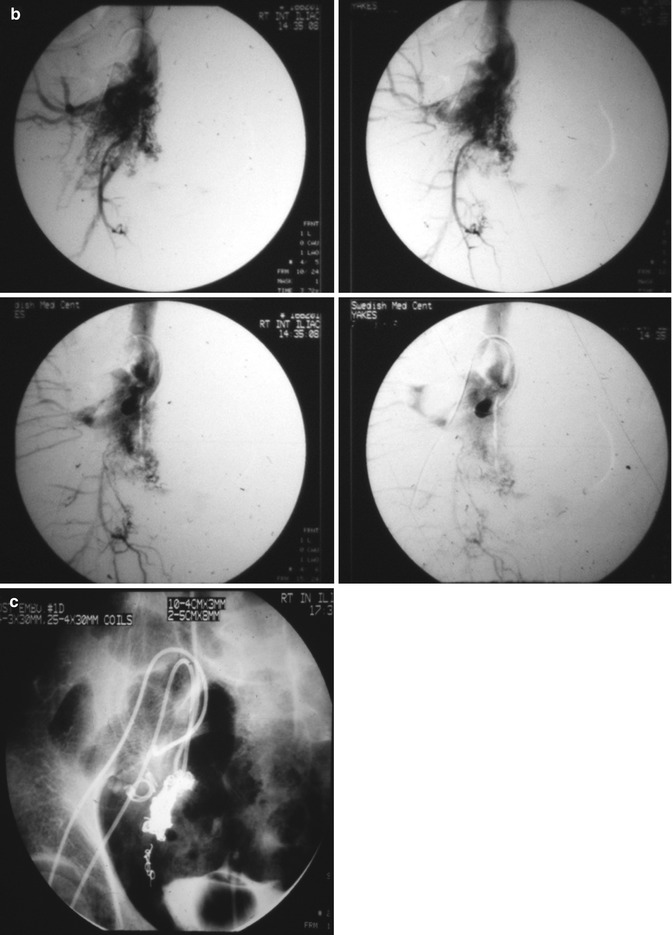
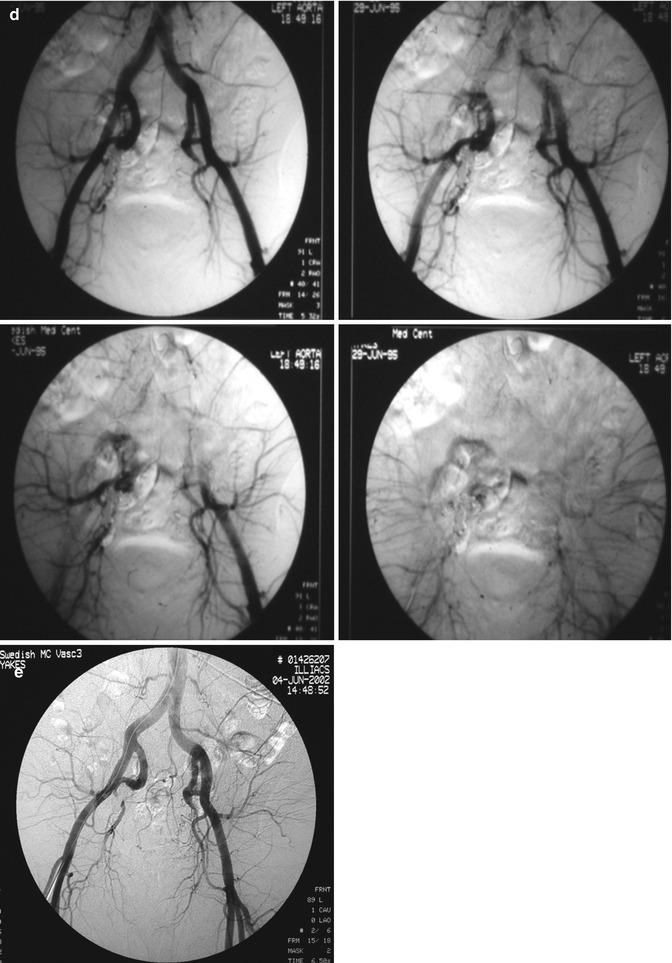
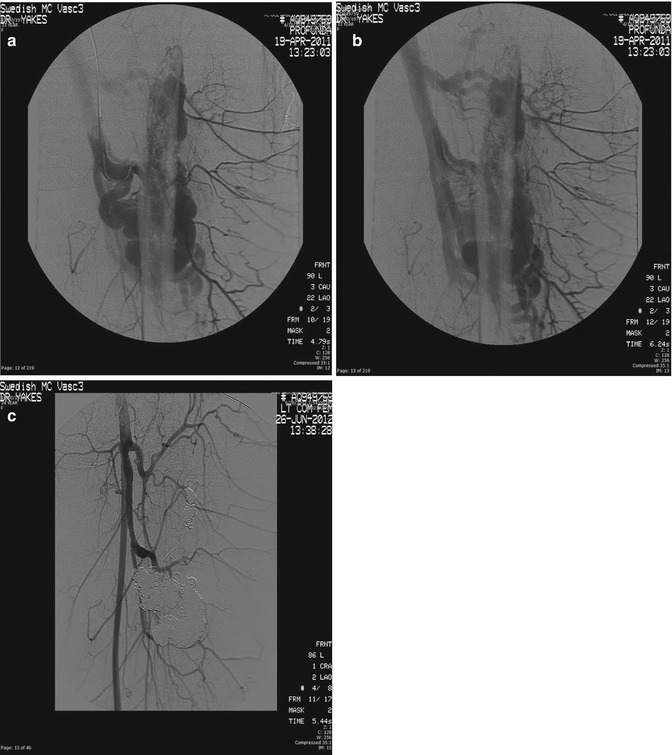



Fig. 33.3
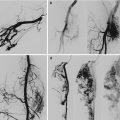
Example of Yakes Type IIIa AVM angioarchitecture with multiple in-flow arteries/arterioles and single out-flow vein physiology. The vein wall is the “nidus” in this AVM type. Multiple Right Internal Iliac Artery branches supply this right pelvic AVM. (a) 32 year old male with right pelvic AVM with single outflow vein drainage towards Right Internal Ilaic Vein. Arterial supply is from multiple Right Internal Iliac Artery branches. Because of the diffuse innumerable small arteries suppling the AVM vein aneurysm wall, transarterial ethanol embolization is not possible. Normal structures could potentially be embolized and resultant nerve damage, pelvic organ damage, tissue necrosis, etc., could result (b) AP selective Right Internal Iliac Artery arteriogram demonstrating innumerable small arterial connections to the single out-flow aneurysmal vein. Superselective catheter positioning for transarterial embolization is not possible. A retrograde venous approach must be employed to treat this Yakes Type IIIa AVM. (c) AP pelvis spot film demonstrating arterial catheter in Right Internal Iliac artery, and the retrograde vein catheter placed centrally within the AVM vein aneurysm with the resultant deposition of multiple coils in the vein aneurysm to treat this Yakes Type IIIa AVM (d) AP pelvis arteriogram immediately post-coil embolization demonstrating total occlusion of the right pelvic AVM. No residual arteriovenous shunting is present. All normal arteries remain intact post-coil embolization without complication. (e) AP pelvis follow-up arteriogram 7 years post-retrograde vein coil embolization demonstrating long-term cure of the right pelvic AVM. Again, this technique is curative in Type IIIa and Type IIIb AVMs

Fig. 33.4
Example of Yakes Type IIIb AVM typified by multiple inflow arteries/arterioles shunting into the aneurysmal vein with multiple out-flow veins. The “nidus” is the vein wall with the innumerable AV connections. (a) Left soft tissue and intraosseous left Femur AVM. Multiple arterial inflow branches from the Left Profunda Femoris Artery into the AVM. The arterial/arteriolar inflow has many parenchymal branches and also provides vascular supply to the AVM. (b) Venous phase of the Left Profunda Femoris arteriogram demonstrating the vein aneurysms and multiple out-flow vein physiology. To treat this Yakes Type IIIb AVM, multiple veins must be occluded to completely treat this AVM. Transarterial embolization is difficult to perform in that tissue necrosis could occur due to the many parenchymal arterial branches also arising from these multiple AVM feeding branches. (c) Left Common Femoral arteriogram at over one year follow-up demonstrating cure of the soft tissue and intraosseous AVM components. Note the multiple coil placements required to treat the multiple out-flow vein compartments that are present in Yakes IIIb AVMs
Yakes Type IV angioarchitecture has innumerable micro-AV connections (with lowered vascular resistance) infiltrating an entire tissue but with concurrent normal vascular structures possessing nutrient capillary beds (with normal vascular resistance) to supply and drain the tissue that is diffusely infiltrated to allow this tissue to survive and not be devitalized. The postcapillary veins compete with AVF outflow veins that are arterialized (hypertensive) (Fig. 33.5) and cause the resultant nonhealing pathology. This entity has not been described in the world’s literature [9–22].
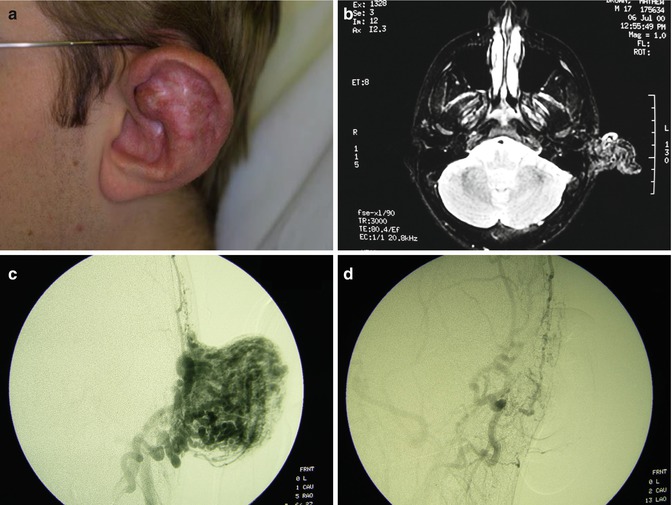
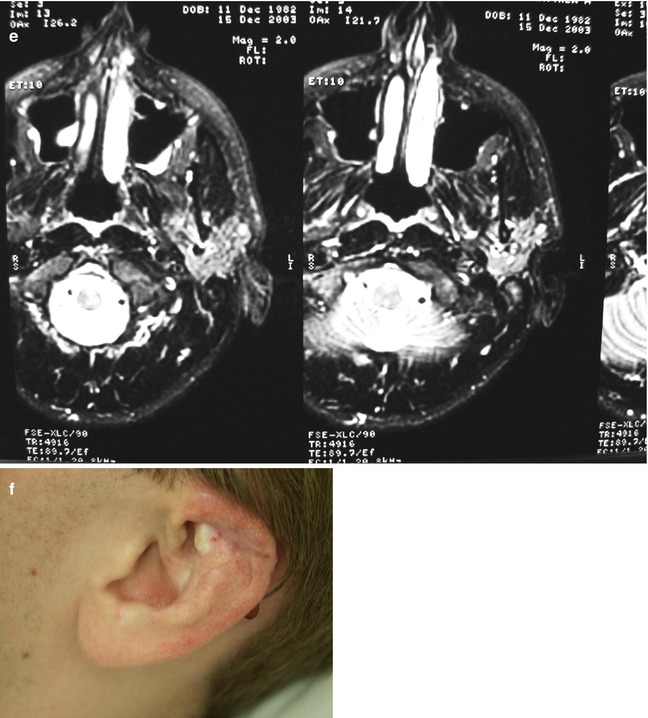


Fig. 33.5




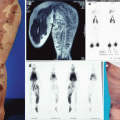
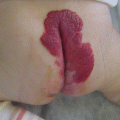
Example of Yakes Type IV AVM typified by total infiltration of a tissue with innumerable micro-fistulas and innumerable outflow-veins. Capillaries are admixed with the innumerable fistulas throughout the tissue involved with this “infiltrative” form of AVM. (a) 19 year old male with progressively enlarging left ear over the last 5 years. Now has developed intermittent ulcerations, infections, and hemorrhages (Shobinger III stage). (b) T-2 weighted axial MR demonstrating an enlarged left ear with flow-voids totally infiltrating the entire ear and cartilages. The enlargement of the abnormal ear tissues is apparent. (c) AP External Carotid arteriogram demonstrating diffuse vascular infiltration of the left ear with innumerable micro-fistulae shunting into abnormal arterialized veins. The arteriogram mirrors the findings noted on the MR with ear enlargement and total micro-fistulous AVM infiltration and AV shunting evidenced by the MR flow-voids. (d) AP Left External Carotid arteriogram at 4 year follow-up demonstrating persistent cure of the left ear AVM. Note the normal vascularity that is now present and the total absence of any residual AV shunting post-endovascular ethanol sclerotherapy (e) Axial MR T-2 weighted at 3 year follow-up demonstrating shrinkage of the left ear and normalization of the vascularity with total absence of the innumerable flow-voids previously present on the pre-treatment MR. (f) 4 year clinical follow-up of the left ear. A successful plastic surgery procedure was performed on the superior aspect of the left ear after the ear AVM was totally ablated and cured. The ear now has a more normal contour. Note the normalization of the skin color, no residual ruborous venous hypertensive skin changes are present
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree