Oropharynx
The incidence of oropharyngeal carcinoma is increasing. This is in contrast to the decreasing incidence of head and neck cancer arising in other anatomic sites. The classic etiologic factors of tobacco abuse and alcohol use continue to play a significant role. However, a significant increase in rates of oropharyngeal cancers in nonsmokers and nondrinkers caused by oncogenic human papillomaviruses (HPVs) is occurring, predominantly among men. Although both HPV–associated and HPV-unassociated malignancies are classified as squamous cell carcinomas, the behavior of these cancers markedly differs as HPV-associated cancers have a significantly more favorable prognosis after the delivery of standard treatments. The recognition that HPV-associated oropharyngeal cancer is a distinct clinical entity, as well as the impact of standard therapies on speech, swallowing, degustation, and psychological well-being, has led to significant multidisciplinary interest in defining different treatment paradigms for HPV-associated and HPV-unassociated oropharyngeal cancers. Treatment recommendations for these two clinical entities remain the same, however, until ongoing investigations are completed.
Based on the critical function of the oropharynx in speech and swallowing, the treatment of oropharyngeal carcinomas can significantly impact patient quality of life. Appropriate treatment strategies should focus on maintaining high cure rates while minimizing long-term, treatment-induced functional morbidity. Early-stage oropharyngeal cancers are managed with single modality therapy (radiation or surgery), with the choice based on anticipated posttherapy consequences. Treatment of locoregionally advanced tumors involves multiple modalities with the use of either concomitant chemotherapy and radiotherapy or surgery followed by adjuvant radiotherapy with or without chemotherapy based on pathologic risk factors.
Novel surgical and radiation delivery modalities, as well as molecularly targeted chemotherapeutics, are the current focus of clinical investigation, with an intent to maximize the therapeutic index for HPV-associated oropharyngeal cancers. Transoral surgical advances including laser and robotic interventions have the potential to reduce morbidity. Advances in radiotherapy allow for improved dosing to the primary tumor and involved nodes while reducing radiation exposure of normal tissues, particularly the parotids and pharyngeal constrictors. Further studies are needed to more completely integrate molecularly targeted agents into the treatment of oropharyngeal cancers.
 EPIDEMIOLOGY
EPIDEMIOLOGY
Oropharyngeal cancers account for approximately 10% of the annual worldwide incidence of head and neck squamous cell carcinomas. The incidence of oropharyngeal cancer differs significantly by geography.1 In the United States, the annual incidence of oropharyngeal squamous cell carcinoma is 4.8 in 100,000,2 which is similar to other developed countries. This rate increased by 28% from 1988 to 2004, largely because of the 225% increase in HPV-associated oropharyngeal cancer, whereas HPV-unassociated oropharyngeal cancer declined by 50% over the same time period.3 The incidence of oropharyngeal cancer in developing countries is lower at approximately 3 in 100,000.1 This rise in developed countries is unique because other mucosal head and neck cancer incidences have decreased over this same time period. The putative cause is the increasing incidence of HPV-associated cancers, which is discussed in detail later. Worldwide, the majority of oropharyngeal cancers remain attributable to tobacco smoking and/or the ingestion of excessive amounts of alcoholic beverages. For these cases, incidence rates are generally higher for men than women (4:1), who are diagnosed more commonly in the sixth and seventh decades of life.
 HUMAN PAPILLOMAVIRUS–ASSOCIATED OROPHARYNGEAL CANCER
HUMAN PAPILLOMAVIRUS–ASSOCIATED OROPHARYNGEAL CANCER
Human Papillomavirus
HPV is a circular, double-stranded DNA virus, first determined to be oncogenic when it was found to be the associated with cervical cancer in 19834 and subsequently established as a significant human carcinogen in 1996.5 To date, approximately 150 HPV types have been identified. HPV subtypes are classified as high or low risk based on epidemiologic associations with cervical cancer in case-control studies.6 HPV 16 is the most common HPV type identified in human tumors and is associated with more than 90% of all HPV-associated related oropharyngeal cancers.7 Infection with HPV 16 confers an approximate 14-fold increase in risk for oropharyngeal cancer.8
The HPV genome encodes three oncoproteins (E5, E6, and E7), in addition to regulatory genes (E1 and E2) as well as capsid protein genes (L1 and L2). Oncogenesis is primarily mediated via the E6 and E7 proteins. HPV E6 complexes with E3 ubiquitin ligase and E6-associated protein, promoting ubiquitin-mediated destruction of p53. Loss of cellular p53 function results in dysregulation of the G1/S and G2/M checkpoints. An E7/cullin 2 complex ubiquitinates the Rb protein, resulting in loss of G1/S checkpoint control.9 E7 is believed to be the major transforming oncogene during early carcinogenesis, with E6 functioning later.10 A diagram of the pathways affecting the malignant transformation of keratinocytes by HPV is shown in Figure 45.1. Although E6 and E7 oncoprotein function is necessary for development of an HPV-associated malignancy, it is not sufficient. It is believed that as yet undefined genetic events are required for HPV malignant transformation.11
Several different techniques are used to detect HPV in oropharyngeal cancer biopsy specimens. The gold standard is demonstration of HPV E6/E7 in clinical specimens. However, this approach is clinically impractical because it is very difficult to detect viral RNA from cytologic fluid and paraffin embedded tissues. Polymerase chain reaction (PCR) of HPV DNA is a technique with high sensitivity but low specificity, as cross contamination or transcriptionally inactive DNA can be detected. In situ hybridization (ISH) uses oligonucleotide probes designed to anneal to complementary HPV DNA in the tumor specimen. Advantages of this technique include localization of DNA within the tumor specimen and allow for identification of a single viral copy.12 A consequence of HPV E7-mediated Rb inhibition is induction of demethylases resulting in expression of p16INK4A, an upstream tumor suppressor cyclin-dependent kinase inhibitor.13 Immunohistochemistry staining for p16INK4A is frequently used as a surrogate for HPV status. There is a small (7%) discordance between HPV ISH and p16INK4A IHC (approximately 7%), which is likely related to a combination of infection with non-HPV-16 subtypes or low viral copy numbers not detectable by IHC and true p16-positive/HPV-negative cases.
FIGURE 45.1. Diagram of malignant transformation in keratinocytes caused by the HPV oncoproteins E6 and E7. Clockwise from A, ubiquitination by E7 and the cullin 2 ubiquitin ligase complex leading to pRb degradation (23, 25, 56, 57); B, interaction between E7 and p27Kip1 resulting in inhibition of cell cycle arrest contributing to carcinogenesis (58); C, interaction between E7 and p21Cip1 resulting in inhibition of cell cycle arrest contributing to carcinogenesis (31, 59); D, ubiquitination by E6 and ubiquitin ligase E6AP leading to p53 degradation (19–21); E, increased expression of p16INK4A by a consequent of feedback loops from the absence of pRb function (42); and F, degradation of NFX1, a transcriptional repressor of hTERT, by association with E6/E6AP resulting in hTERT activation and cellular immortalization (60). (From Chung CH, Gillison ML. Human papillomavirus in head and neck cancer: its role in pathogenesis and clinical implications. Clin Cancer Res 2009;15(22):6758–6762, with permission.)
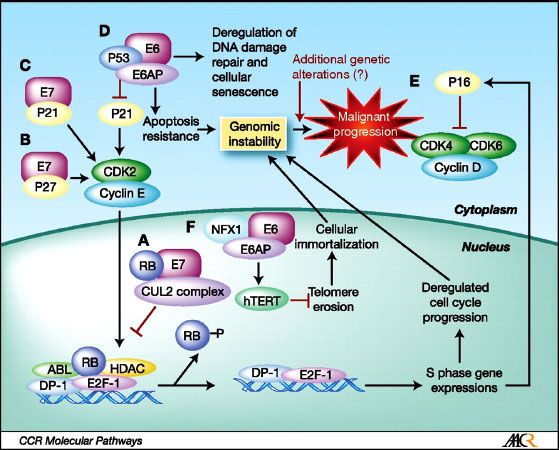
TABLE 45.1 HUMAN PAPILLOMAVIRUS STATUS AND SURVIVAL OUTCOMES IN PROSPECTIVE TRIALS

Clinical Characteristics of Human Papillomavirus–Associated Oropharyngeal Cancer
HPV-associated oropharyngeal cancers are more likely to occur among men and than women (3:1), most of whom (80%) will not have a smoking history. These cancers are more common among white individuals than other races, are diagnosed in individuals who are 5 to 10 years younger than HPV-unassociated oropharyngeal cancers, and are associated with higher socioeconomic status. Furthermore, when compared to patients with HPV-unassociated cancers, these patients are more likely to be married, college educated, and have a median income of more than $55,000. The use of marijuana also elevates odds of HPV-associated oropharyngeal cancer.14 Analogous to cervical cancers, patients with HPV-associated oropharyngeal cancer have been associated with certain sexual behaviors. These include high number of vaginal or oral sex partners, infrequent condom use, engagement in casual sex, and early age of first intercourse.15 Whether or not HPV acts in a synergistic manner with tobacco or alcohol exposure in patients to increase the risk of HPV-associated oropharyngeal cancer is a matter of ongoing controversy.
HPV-associated oropharyngeal cancers are characterized frequently as poorly differentiated, nonkeratinizing, or basaloid in histopathology.16 HPV-associated and HPV-unassociated carcinomas are also different with regard to molecular alterations. HPV-associated oropharyngeal cancers demonstrate wild-type p53, p16 expression, and infrequent amplification of cyclin D, whereas the converse is true for HPV-unassociated cancers. A subset of HPV-associated oropharyngeal cancer patients with more extensive smoking histories will have tumors exhibiting TP53 mutations, higher epidermal growth factor receptor (EGFR), and Bcl-xL expression and have outcomes similar to those of HPV-unassociated patients.9
Response of Human Papillomavirus–Associated Oropharyngeal Cancer to Standard Therapy
Patients with HPV-associated oropharyngeal cancers have significantly better outcomes compared to HPV-unassociated oropharyngeal tumors.17,18 In a reanalysis of Radiation Therapy Oncology Group (RTOG) 0129, a randomized study comparing cisplatin administered with either accelerated concomitant boost radiotherapy or conventionally fractionated radiotherapy, HPV status was independently associated with improved outcomes. Three-year overall survival was 82% in HPV-positive patients compared with 54% in HPV-negative patients. Even after adjustment for age, tumor stage, nodal stage, treatment assignment, and tobacco use, HPV status independently predicted for improved survival (hazard ratio [HR] 0.42, 95% confidence interval [CI] 0.27 to 0.66). Additionally, locoregional progression (13.6% vs. 24.8%) and progression-free survival (71.8% vs. 50.4%) were significantly improved in HPV-positive cases. Other large cooperative group studies have demonstrated similar findings.18,19 It is important to realize that the prognostic significance of HPV was independent of the chemoradiotherapy platform and applies to treatment with radiotherapy alone,20 as shown in Table 45.1. In fact HPV-related tumors have a better prognosis regardless of the treatment modality (surgery, radiotherapy, or chemoradiotherapy) used.
TABLE 45.2 ANATOMIC BOUNDARIES OF NECK NODE LEVELS

TABLE 45.3 RADIOGRAPHIC BOUNDARIES OF NECK NODE LEVELS
 ANATOMY
ANATOMY
The oropharynx is contiguous with the oral cavity anteriorly, the larynx and hypopharynx posterior-inferiorly, and superiorly with the nasopharynx. Three main subregions compose the oropharynx including the tonsil, base of tongue, and soft palate. Normal function of the oropharynx is critical for speech and swallowing.
The tonsillar region contains the anterior and posterior tonsillar pillars as well as the palatine tonsil. The palatine tonsils are lymphoid aggregates incompletely encapsulated with a keratinized stratified squamous epithelial mucosal lining positioned in the tonsillar bed, which is a part of the tonsillar cleft between the anterior (palatoglossal) and posterior (palatopharyngeal) tonsillar pillars.
The base of tongue comprises the posterior third of the tongue and is bounded anteriorly by the circumvallate papillae, sitting in front of the sulcus terminalis. The base of tongue is bounded posterior-inferiorly by the hyoid and epiglottis and laterally by the glossopharyngeal sulci. Underlying the mucosa of the base of tongue are lymphatic nodules collectively known as the lingual tonsil. The vallecula is a 1-cm mucosal strip that serves as a transition between the base of tongue and epiglottis and is considered a part of the base of tongue. The sensory innervation of the base of tongue is via the glossopharyngeal nerve (cranial nerve [CN] IX) with a small aspect of the base of tongue supplied by the internal laryngeal nerve (CN X).
The soft palate is a fibromuscular structure bounded anteriorly by the hard palate, laterally coursing into the anterior tonsillar pillars and posterior-inferiorly forming a free edge, and the midline uvula. The soft palate is composed of five muscles (levator veli palatini, tensor veli palatini, palatoglossus, palatopharyngeus, and musculus uvulae) posteriorly and the palatine aponeurosis an expanded tendon of the tensor veli palatini anteriorly. The muscles of the soft palate are supplied through the pharyngeal plexus (which is composed of the pharyngeal branches of CNs IX and X, as well as sympathetic branches from the superior cervical ganglion, except for the tensor veli palatini, which is supplied by CN V2). The sensory supply is from CN IX.
The oropharynx serves many functions, including that of degustation, respiration, and speech. Advanced tumors arising in the oropharynx can infiltrate muscles and nerves, thus significantly impeding these functions. A major goal of successful therapy is to limit the impact of the treatment on long-term function.
 ROUTES OF SPREAD
ROUTES OF SPREAD
Primary routes of spread for oropharyngeal cancers include direct extension and lymphatic spread, with hematogenous metastases being less common. Oropharyngeal cancers have a predilection for submucosal extension, often visualized as raised erythematous regions without distinct borders or ulceration. This can best be appreciated by direct visualization rather than on radiographic imaging.
Lymphatic Spread of Oropharyngeal Cancer
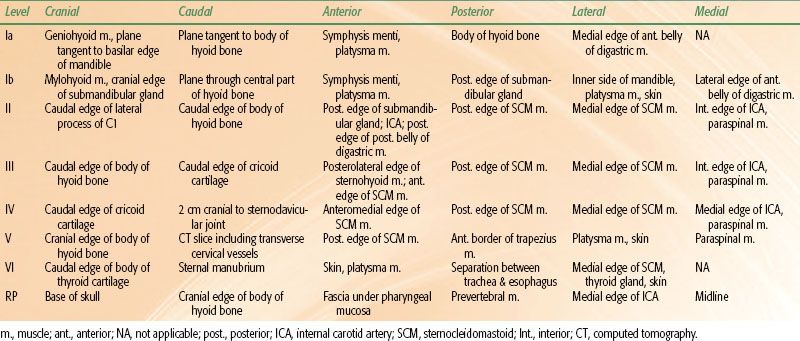
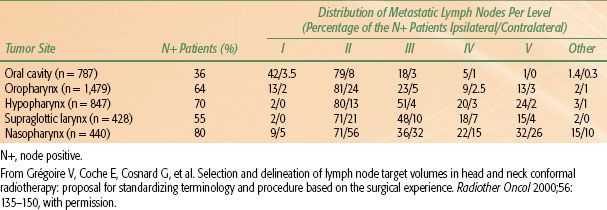
The lymphatic drainage of the oropharynx and the neck was first described by Rouviere21 in 1938 and has since been refined by others.22 Originally grouped by lymph node chains located in particular anatomic regions, nodal groups are now classified by the level system23 with the location of lymph nodes in the neck being defined by surgical-anatomic landmarks (Table 45.2). Recently, this system (levels I to VI) was refined with the addition of sublevels (Ia/Ib, IIa/IIb, and Va/Vb) (as shown in Table 45.2), also incorporating radiologically defined landmarks24 (as shown in Table 45.3 and Fig. 45.2).
The most common location for lymph node metastases from oropharyngeal cancers is the ipsilateral level II. The probability of lymphatic (regional) metastasis is related to the size and location of the primary tumor within the oropharynx. The typical order of metastatic progression is systematic, from the upper jugular chain nodes superiorly (level I/II; first echelon), to mid-cervical (level III), and to lower cervical nodes (level IV), inferiorly. In large series of oropharyngeal cancer patients, isolated skip metastases are rare (0.3%), and level I or V involvement is usually associated with the involvement of other levels. Additionally, tumors encroaching or crossing midline or involving the posterior pharyngeal wall exhibited a higher propensity for bilateral lymphadenopathy.
Knowledge of the probability of occult pathologic lymphadenopathy for each involved oropharynx anatomic subsite and extent of disease is critical to modern radiotherapy and surgery planning, as selective neck dissections and limited radiotherapy volumes are the norm. Standardized contouring atlases have been published to aid clinicians in development of appropriate radiotherapy volumes to cover potential occult microscopic lymphatic spread in the N0 neck.25–27 Additionally, information about probabilities of occult pathologic lymphatic involvement has been compounded from series of patients undergoing elective neck dissection.28 The use of this knowledge to develop appropriate radiotherapy volumes will be discussed in more detail in the radiotherapy volumes section. The rate of pathologic lymphadenopathy for the pathologically involved neck is outlined in Table 45.4.
FIGURE 45.2. Schematic diagram indicating the location of the lymph node levels in the neck based on anatomic boundaries. (Used with the permission of the American Joint Committee on Cancer (AJCC), Chicago, Illinois. The original source for this material is the AJCC Cancer Staging Handbook, Seventh Edition [2010] published by Springer Science and Business Media LLC, www.springerlink.com.)
TABLE 45.4 DISTRIBUTION OF CLINICAL METASTATIC NECK NODES FROM HEAD AND NECK SQUAMOUS CELL CARCINOMAS
Distant Metastatic Spread of Oropharyngeal Cancer
Distant metastatic spread in oropharyngeal cancer is relatively uncommon, affecting approximately 15% of all patients during the course of their disease.29 The most common locations for distant metastatic spread of oropharyngeal cancers are the lung parenchyma,29 followed by osseous and hepatic metastases. Metastases are more common in patients presenting with locoregionally advanced or recurrent tumors, with the risk increasing with primary tumor stage as well as the burden of pathologic lymphadenopathy (N2-N3 disease).29,30 Extranodal extension, lower cervical pathologic lymphadenopathy (level IV), and lymphovascular invasion have also been associated with increased rates of distant metastases.31
Metastatic deposits within the lung parenchyma typically appear radiographically as well-circumscribed, peripherally located nodules. Care should be taken to differentiate between pulmonary metastases and primary pulmonary malignancies, characterized as spiculated irregularly shaped masses commonly associated with hilar and mediastinal lymphadenopathy, given their differing prognostic implications. This distinction often is impossible, even after biopsy, as both can be of squamous cell histology. In such instances, physically fit patients should be given the benefit of the doubt and treated as if they have two separate primary tumors. In patients with limited pulmonary metastases, who are technically resectable and fit for surgery, resection of pulmonary oropharyngeal cancer metastases may improve survival.32–34 For nonsurgical candidates, hypofractionated image-guided radiotherapy to all known metastatic sites can result in long-term disease control and should be considered for patients with limited metastatic disease.35
 CLINICAL PRESENTATION
CLINICAL PRESENTATION
Oropharyngeal cancers present with a constellation of symptoms that depend on the location of the primary tumor, invasion of nearby organs, and extent of nodal disease. Often, patients will present with a painless neck mass, which is usually mobile, firm, and nontender but can be fixed, indicating extranodal extension and invasion into surrounding structures. Such masses are frequently treated with an initial course of antibiotics; however, persistence or growth in this context mandates further evaluation. Some patients complain of a deep-seated otalgia located within the auditory canal. This is mediated via irritation of the glossopharyngeal nerve (CN IX) with referral via the petrosal ganglion to the tympanic nerve of Jacobson. Regurgitation of foods can occur with invasion of the soft palate, inhibiting its ability to elevate during swallowing. Trismus is seen with more advanced tumors and reflects invasion of the pterygoid fossa and/or musculature. Odynophagia and dysphagia are other common presenting symptoms that occur with invasion into the pharyngeal musculature or obstruction by pathologic lymphadenopathy.
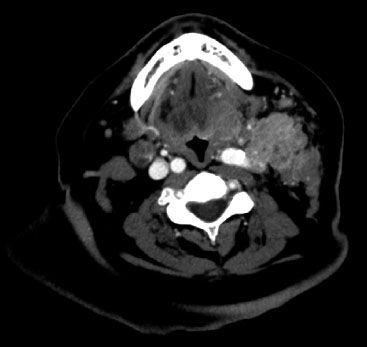
FIGURE 45.3. Diagnostic computed tomography image demonstrating locoregionally advanced oropharyngeal cancer with extensive ipsilateral cervical lymphadenopathy.
 DIAGNOSTIC EVALUATION
DIAGNOSTIC EVALUATION
Physical Examination
A complete examination of all mucosal head and neck sites should be performed in any patient with a known or suspected diagnosis of oropharyngeal cancer. This process not only characterizes the primary tumor but also evaluates for other malignancies given the high propensity for second primary upper aerodigestive tract tumors. A thorough physical examination is essential for diagnosis and understanding of the complete extent of disease, and it helps to guide the surgeon on the choice of optimal biopsy site. Inspection of the oropharynx should be performed under adequate illumination and be well practiced, systematic, and reproducible. Following examination of the oral cavity, where attention should be directed to the number and health of the patient’s teeth and to the mucosal sites, one should closely examine the anterior tonsillar pillars, the tonsillar fossae, and posterior tonsillar pillars followed by the soft palate. Proper exposure can be achieved either with gloved index fingers or with two disposable tongue depressors used in unison. Palpation of the tonsillar fossa and the base of tongue should be performed because these locations can harbor occult primary tumors, with the base of tongue performed at the completion of the examination owing to its propensity to trigger the gag reflex. Direct visualization should be followed by fiberoptic examination whenever possible, because this allows optimal inspection of the base of tongue, posterior-inferior tonsil vallecula, as well as documenting spread to laryngeal and pharyngeal subsites. Fiberoptic examinations should be recorded and compared to assess response during the course of therapy. Indirect mirror examination is less informative than fiberoptic evaluation; however, it should be performed if fiberoptic capabilities are not available.
Oropharyngeal tumors often appear as ulcerated masses, with surrounding erythema, neovascularization, and mucositis. Tenderness, evidence of recent bleeding, obstruction of the airway, skin invasion, alteration of gag reflex, and extent of trismus (measured from upper to lower incisors) should be documented. Bulging of the parapharyngeal space should also be noted because this could represent retropharyngeal lymphadenopathy.
Careful examination of the neck is also important for staging and management. Palpation of the neck should focus on neck levels defined by standard anatomic relationships. The neck should gently be turned to the side while being examined to relax the sternocleidomastoid muscle, which facilitates the detection of smaller involved lymph nodes. Care should be taken not to palpate too firmly in older patients or those with known vascular disease, as aggressive carotid massage can be associated with syncope. Lymph nodes should be recorded in terms of the level in which they arise, their size, and the character of their firmness, as well if they are fixed and whether or not they penetrate and involve the skin.
Confirmatory biopsy of the primary site should be performed. Adequate exposure is usually possible for in-office biopsies of the proximal oropharynx. Posterior oropharyngeal tumors are often biopsied under general anesthesia in the operating room, often as part of a comprehensive examination under anesthesia as well as comprehensive endoscopic evaluation (laryngoscopy, bronchoscopy, and esophagoscopy).
Computed Tomography
Computed tomography (CT) imaging of the head and neck with intravenous contrast should be performed for all newly diagnosed oropharyngeal cancer patients to assess the extent of primary tumors and to determine the presence or absence of cervical lymph node metastases. Scan slice thickness <5 mm is desirable to optimize the detection of smaller pathologically involved lymph nodes and to provide the best anatomic delineation of both primary and nodal disease. Pathologically involved lymph nodes are characterized on CT imaging as those that are enlarged, enhance with contrast, and have a necrotic center. Primary tumors appear as contrast-enhancing masses, distorting normal anatomic relationships. Whereas ulceration and invasion into surrounding organs are readily assessed, submucosal spread is often difficult to characterize with CT. As multiplanar image reconstruction is routinely available, lymph node and primary tumor size should be measured in both longitudinal and cross-sectional dimensions to more accurately stage patients. Unfortunately, dental artifact on CT imaging may often obscure complete visualization with neutral head position, requiring further scanning with an adjusted head position. Thoracic CT should be performed routinely to assess for pulmonary spread of oropharyngeal cancer patients with N2 or greater nodal disease, as well as those with advanced primary tumors, given the risks of pulmonary metastases described previously. A diagnostic CT image of a patient with locoregionally advanced head and neck cancer is shown in Figure 45.3.
Positron Emission Tomography
Positron emission tomography (PET) and/or PET/CT imaging incorporating tumor physiology in conjunction with anatomic information are now routinely recommended for the initial staging of oropharyngeal cancer patients. From a practical standpoint, PET-based imaging can assess not only the locoregional burden of disease but also detect and quantify distant metastases. For oropharyngeal cancer patients specifically, the ability to detect clinically and radiographically occult pathologic cervical lymphadenopathy renders PET a powerful clinical tool, as ipsilateral radiotherapy volumes are used in specific circumstances.36 Although commonly reported as the maximum standard uptake value (SUVmax), alternative measurements such as SUVmean may have greater prognostic value.37 The utility of PET/CT for oropharyngeal cancer patients demonstrates high sensitivity approaching 100%, although only about 60% specificity for pathologically proven tumor. Clinical status and knowledge of prior procedures is critical to PET interpretation, because recent biopsies and infections can cause artificially elevated metabolic activity.
Magnetic Resonance Imaging
Magnetic resonance imaging (MRI) can be a useful imaging tool for oropharyngeal tumors. Squamous cell carcinoma appears as low signal in T1 MRI and corresponding high signal in T2 sequences. The ability of MRI to differentiate tumor from soft tissues is particularly useful when determination of the extent of base of tongue or oral tongue invasion is needed. Additionally, MRI is useful in patients with compromised renal function who are not able to receive iodine-based CT contrast agents.
TABLE 45.5 TNM STAGING SYSTEM FOR OROPHARYNGEAL CANCER

TABLE 45.6 STAGE GROUPING FOR OROPHARYNGEAL CANCER

 PATHOLOGIC CLASSIFICATION
PATHOLOGIC CLASSIFICATION
Squamous cell carcinomas are the most common histologic subtype comprising more than 95% of all oropharyngeal cancers. Uncommonly, minor salivary gland and mesenchymal tumors can affect this region. Given the proportionally high content of lymphoid tissue in the region within Waldeyer’s ring, malignant Hodgkin and non-Hodgkin lymphomas also arise in this region. This chapter will discuss only squamous cell and related (poorly differentiated and lymphoepithelioma) histologic subtypes. Malignant lymphomas are discussed in Chapters 77–78. Minor salivary gland tumors are discussed in Chapter 43, and sarcomas are discussed in Chapters 48 & 83.
 STAGING
STAGING
Staging for oropharyngeal cancer is based on the American Joint Committee on Cancer (AJCC)/Union for International Cancer Control (UICC) system, shown in Table 45.5. Clinical staging is based on all available history, physical examination, endoscopic, radiographic, metabolic, and scintigraphic data. For all subsites, the size of the primary tumor contributes to the T-stage, with T1 being <2 cm, T2 >2 cm but <4 cm, and T3 >4 cm. T4a describes tumors invading the larynx, extrinsic muscles of the tongue, medial pterygoid, hard palate, or mandible. T4b disease describes oropharyngeal tumors invading the lateral pterygoid, pterygoid plates, lateral nasopharynx, skull base, or surrounding the carotid artery. Nodal staging for each of the subsites is the same: N0 indicates no clinical or radiographic evidence of pathologic lymphadenopathy; N1 indicates a single lymph node <3 cm in the ipsilateral cervical chains; N2a indicates a single lymph node >3 cm and <6 cm in the ipsilateral cervical chain; N2b indicates multiple ipsilateral cervical lymph nodes all <6 cm in size; N2c indicates bilateral pathologically involved cervical lymph nodes with the largest node <6 cm; and N3 indicates the presence of at least one lymph node >6 cm. Distant metastatic disease is classified as M1.
Stage grouping is shown in Table 45.6. Stage I comprises T1N0 tumors, and stage II is made up of T2N0 tumors. Stage III includes patients with T3N0–1 or T1-T2N1. Stage IV is divided into three subgroups: stage IVa is made up of patients with T4aN0–2a-c, T1–3N2a-c tumors; stage IVb disease basically describes patients who are technically unresectable, including patients with an extensive primary tumor (T4b) or those with any primary stage who have extensive lymphadenopathy (T any N3); stage IVc disease is reserved for patients with distant metastases.
 MANAGEMENT STRATEGIES
MANAGEMENT STRATEGIES
Functional organ preservation with minimal toxicity is the management goal for all oropharyngeal cancer patients. Based on AJCC stage, patients are usually grouped into two different treatment groups to help guide therapy decisions. Those with locally confined disease (stage I and stage II tumors) are considered as early stage, whereas those with stages III and IV (nonmetastatic) disease are considered as having locoregionally advanced disease. For all subsites, early-stage tumors are usually well controlled with a single local modality, either radiotherapy or surgery. Selection of local modality should be based on the primary tumor size, extent of local spread, and subsite involved. Small tumors of the tonsil and small exophytic tumors of the base of tongue can be well managed surgically, whereas the morbidity of surgery on the soft palate favors radiotherapy. For locoregionally advanced disease, two appropriate treatment strategies are used: (a) either surgery followed by radiation therapy with or without chemotherapy based on pathologic risk factors or (b) radiotherapy usually given with chemotherapy.
 SURGICAL TECHNIQUES, APPROACHES, AND RESULTS
SURGICAL TECHNIQUES, APPROACHES, AND RESULTS
Base of Tongue
Surgery plays a limited role in the management of base of tongue tumors given the inherent morbidity of a near-total or total glossectomy, which is required for large and/or midline tumors. For select, well-lateralized base of tongue tumors with minimal cervical lymphadenopathy, a partial glossectomy can be performed. Given the high propensity for occult microscopic nodal involvement, bilateral cervical lymph node dissection is often performed. Base of tongue tumors in close proximity to the laryngeal apparatus, such as those arising in the vallecula, often require a supraglottic or total laryngectomy to achieve adequate margins of resection.
Traditional surgical approaches for base of tongue tumors include the midline mandibulotomy (splitting the lip, mandible, and oral tongue midline), the lateral mandibulotomy (dividing the mandible near the angle and approaching the base of tongue from the side), and the floor drop procedure (elevating the inner periosteum from the mandible from angle to angle, which releases the entire floor of mouth and oral tongue into the neck, exposing the base of tongue).
Tonsil Cancers
For small (<1 cm) early-stage tonsil cancers confined to the anterior pillar, a wide local excision can achieve adequate tumor-free margins, whereas tumors involving the palatine tonsil often require a radical tonsillectomy. For both of these situations, the tonsil is approached transorally, with primary closure. Larger tumors with extension onto the tongue, onto the mandible or into surrounding tissue often require a composite resection, usually including resection of the tonsil, tonsillar fossa, pillars, a portion of the soft palate, tongue, and mandible. For tumors not adjacent or adherent to the mandible, a midline mandibulotomy approach is used. For tumors adherent to the mandible, a partial mandibulectomy is used. Defects are often closed with a myocutaneous flap. Complications from surgery depend on the extent of resection, with impairment in swallowing possible by removal of part of the tongue or soft palate.
Soft Palate Cancers
Surgical resection is rarely recommended as initial therapy for soft palate tumors. Resection of the soft palate is often associated with significant reflux into the nasopharynx during swallowing, even with the use of custom prostheses. Additionally, because of the midline location, primary disease spreads bilaterally to the neck with frequency high enough to require elective treatment. However, when surgery is performed, the tumors are approached transorally and a full-thickness wide local resection is performed for tumors limited to the soft palate. A more extensive composite resection is required if disease extends to surrounding structures. Flaps or prostheses are used to preserve velopharyngeal competence. Nasal speech is also often a consequence.
Transoral Surgical Approaches
Transoral surgical approaches, routinely used for limited tonsillar resections, are increasingly being used for other oropharyngeal cancer operations as an alternative to open surgical procedures. By limiting the need for open surgical exposure, these operations can have a quicker recovery time and less morbidity. More recently, endoscopic approaches have been adopted to enhance the utility of transoral surgery. However, limited prospective data support the benefit of transoral operations over traditional approaches. Prospective data are needed to further elucidate the benefits of these surgical advances and better integrate them with the other standard oncologic therapies.
Transoral Laser Surgery
Small series report favorable outcomes for selected patients with stage I through stage IV oropharyngeal tumors treated with transoral laser microsurgery with or without neck dissection, followed by adjuvant radiotherapy or chemoradiotherapy.38–40 Positive margin rates are variable (3% to 24%) and appear to vary based on primary site, being more common in base of tongue tumors. Complications include postoperative hemorrhage (5% to 10%). Temporary tracheostomy placement is relatively common (17% to 30%) and needed for exposure, airway control, or aspiration following extensive resection. High rates of locoregional control following this procedure have been reported, primarily for stage I/II patients (87% to 100%), although for stage III/IV patients, local recurrence is more common (20% to 30%). Swallowing outcomes are favorable with series reporting most patients tolerating a normal diet.40
Transoral Robotic Surgery
The use of a computer-aided interaction between the surgeon and the patient is commonly referred to as robotic surgery. The most common robotic surgical system, the da Vinci Surgical System, is comprised of three surgical instruments and a binocular endoscope controlled by robotic arms and inserted under direct or endoscopic guidance by the surgeon from a patient-side apparatus. The surgeon controls the instruments from a console separated from the patient. The operative environment is visualized virtually, in a three-dimensional (3D) environment created via a computer that links the environment provided by the binocular endoscope to the position of the instruments. The surgeon’s movements are translated into the micromovements of the instruments. The advantages of this system include motion scaling, which can increase precision as well as reduce hand tremor and fatigue. When the system is used for transoral surgeries, an assistant is often positioned by the patient’s head.
There are no prospective randomized studies supporting the use of transoral robotic surgery (TORS) for oropharyngeal tumor resection over conventional surgery. All studies to date are small single-institution series. Proponents of TORS highlight an enhanced visualization of the surgical field over traditional transoral techniques. Some have hypothesized that perhaps local control could be enhanced via TORS debulking with minimal acute sequelae. However, this claim has yet to be tested prospectively. Prospective studies have shown that TORS can be used safely with a low risk of laceration or fracture to a patient.41 In a series of 27 patients with tonsillar cancer who underwent TORS tonsillectomy, morbidity was “acceptable,” including one case of musical bleeding and two cases of moderate trismus; one patient required a tracheostomy, and negative margins were obtained in 25 of 27 patients.42
Until mature prospective multi-institutional series and randomized data are available, the true utility of transoral laser microsurgery and TORS remains unknown. Although early results are favorable and associated with shorter hospital stays, long-term data are needed. Additionally, standard oncologic principles limiting the number of modalities used to minimize treatment related side effects should be carefully considered prior to widespread adoption of the surgical techniques.
 ADJUVANT THERAPY FOLLOWING DEFINITIVE SURGICAL RESECTION
ADJUVANT THERAPY FOLLOWING DEFINITIVE SURGICAL RESECTION
Following surgical resection of oropharyngeal cancers, pathologic features including advanced primary T-stage (T3 or T4), lymphovascular space invasion, perineural invasion, positive margins, multiple pathologically involved cervical lymph nodes, and extranodal extension place patients at high risk for locoregional recurrence.43 In these cases, postoperative radiotherapy (PORT), often in conjunction with chemotherapy, has been shown to reduce the risk of locoregional relapse.44,45,46 PORT was shown in RTOG 73–03 to results in superior locoregional control (70% vs. 58%) when compared to preoperative radiotherapy but did not affect survival.47
Adjuvant Chemoradiotherapy for Oropharyngeal Cancer
The addition of cisplatin-based chemotherapy to PORT has been compared to PORT alone for medically fit head and neck cancer patients of any site in several randomized studies.48–51 All of these studies have demonstrated statistically significant48–50 or strong statistical trends51 for improved locoregional control and disease-free survival with the addition of chemotherapy to PORT. Additionally, two of these studies have demonstrated statistically significant improvements in overall survival,48,49 whereas the other two have shown numerically improved but not statistically significant survival improvements.50,51 A significant portion of patients in these studies had oropharyngeal cancer (European Organisation for Research and Treatment of Cancer [EORTC] 30% and RTOG 43%) generalizing these results to oropharyngeal patients with high-risk pathologic features.
Concurrent Chemotherapy Regimens for Adjuvant Chemoradiotherapy
The optimal chemotherapy regimen delivered with PORT is currently unknown. Schedules of bolus cisplatin 100 mg/m2 were tested in two48,51 randomized studies mentioned earlier, one tested 50-mg weekly cisplatin49 and the other tested cisplatin 20 mg/m2 and 5-fluorouracil (5-FU) 600 mg/m2 days 1 through 5 and days 29 through 33.50 There have been no randomized comparisons of these cisplatin-based schedules. Randomized studies have been attempted to identify the role of carboplatin-based chemotherapy concurrently with PORT compared to PORT alone.52 Unfortunately, these studies closed before accrual goals were met, and no significant benefit was found with the addition of carboplatin to PORT. RTOG 0234 randomized high-risk postoperative patients (positive margin, extranodal extension, and/or ≥2 pathologically involved cervical nodes) to PORT in combination with cetuximab (400 mg/m2 loading dose followed by 250 mg/m2 weekly) and weekly docetaxel 15 mg/m2 or to PORT with cetuximab (400 mg/m2 loading dose followed by 250 mg/m2 weekly) and 30 mg/m2 cisplatin weekly. Results of this randomized phase II study are maturing. Currently, no randomized data support the use of taxanes or cetuximab in the postoperative setting.53 Based on the available data, many consider cisplatin 100 mg/m2 every 3 weeks as the standard.
Adjuvant Radiotherapy Dose
The optimal radiation therapy dose for PORT is also not well defined. Most of the randomized studies demonstrating the benefit of concurrent chemotherapy with PORT used radiotherapy doses of 60 to 66 Gy in 2-Gy daily fractions to high-risk areas (primary tumor bed with positive margin or nodal regions with extracapsular spread). Doses of 50 to 54 Gy in 2-Gy fractions were usually given to areas at risk for microscopic involvement. There is little evidence supporting the higher PORT doses used in these randomized trials over those recommended from the PORT-alone dose-finding studies of 63 Gy for extranodal extension and 57.6 Gy for all others. In three of four randomized studies testing the utility of chemotherapy concurrently with PORT, doses of more than 65 Gy were delivered to high-risk areas.48–51 The fourth study, RTOG 95–01, allowed a dose of 60 Gy with or without an optional 6-Gy boost. As these studies were associated with significant benefits for patients with extracapsular extension and positive margins, we recommend similar dosing schedules.
Postoperative Radiotherapy Treatment Volume
The typical treatment volume used in PORT for head and neck cancer includes the bilateral neck and the primary tumor site. However, it is unclear whether both the neck and primary always need to be within the PORT volume. In those with completely resected primary tumors with negative margins whose sole indication for PORT is pathologic cervical adenopathy, some would direct therapy only to the neck. Additionally, for patients with a positive margin as the sole indication for treatment in the setting of a comprehensive neck surgery without pathologically involved cervical lymph nodes, some would direct treatment to the primary resection bed only. For well-lateralized primary tumors, patterns of progression would suggest that PORT to the ipsilateral neck only may be appropriate.53
 DEFINITIVE RADIOTHERAPY
DEFINITIVE RADIOTHERAPY
For early-stage oropharyngeal cancers, the use of radiation therapy as a single modality is associated with good outcomes and functional preservation.54 Although there is not consensus on the optimal dose fractionation schedule for oropharyngeal cancer patients receiving radiotherapy alone, randomized data55–57 and meta-analyses58,59 support an overall survival benefit with the use of accelerated fractionation or hyperfractionated radiotherapy. Therefore, for oropharyngeal cancer treated with radiotherapy alone, strong consideration should be given to altered fractionation of some sort.
Hyperfractionated Radiotherapy
The benefit of hyperfractionated radiotherapy for oropharyngeal cancer was clearly demonstrated in EORTC 22791, in which patients with T2-3N0-1 non–base of tongue oropharyngeal cancers were randomized to conventionally fractionated radiotherapy at 70 Gy (2 Gy per day) or to 80.5 Gy hyperfractionated at 1.15 Gy twice daily. Hyperfractionated radiotherapy was associated with statistically significant improvements in locoregional control (5-year, 59% vs. 40%). Additionally, there was a trend toward improved overall survival (p = 0.08) particularly in stage III patients.57
Accelerated Radiotherapy
Accelerated radiotherapy has also been shown to benefit oropharyngeal cancer patients; however, this may depend on the exact regimen used. For example, a randomized study comparing an accelerated regimen of 66 to 70 Gy delivered in 2-Gy daily fractions 6 days a week to the same dose delivered 5 days a week with oropharyngeal cancer affecting the majority of patients demonstrated improved locoregional control (42% vs. 30%, p = 0.004), disease-free survival (50% vs. 40%, p = 0.03), and a trend toward improved overall survival (35% vs. 28%, p = 0.07).60 When analyzed as a separate subgroup, pharyngeal primary sites had improved locoregional control (HR 0.6, 95% CI 0.41–0.86).56 Of note, accelerated fractionation improved local control for both p16-positive (HR 0.56, CI 0.33–0.96) as well as p16-negative tumors (HR 0.77, CI 0.60–0.99).61 However, when a more intensive accelerated regimen of 1.8 Gy twice daily to 59.4 Gy was compared to 70 Gy in 2-Gy fractions in stage III/IV head and neck cancer patients, no statistical benefits were seen in terms of locoregional control or overall survival.62 It is unknown if the lack of benefit seen was due to the regimen used or to inclusion criteria because the benefit for acceleration in some randomized studies was less significant for those with stage IV disease as well as those with a larger nodal disease burden.60
Accelerated Versus Hyperfractionated Radiotherapy
For oropharyngeal cancer patients in particular, and head and neck cancer patients in general, it is not known if hyperfractionated or accelerated radiotherapy is superior. The meta-analysis of radiotherapy in carcinoma of the head and neck collaborative group pooled 15 randomized studies (including 6,515 patients) comparing conventionally fractionated radiotherapy to either accelerated radiotherapy or hyperfractionated radiotherapy. Oropharyngeal cancer patients were the largest subsite, representing 44% of all patients (1,585 patients). Altered fractionation radiotherapy regimens were associated with a 3.4% absolute improvement in 5-year overall survival. Heterogeneity in patients included on accelerated and hyperfractionated trials obscure direct comparison, although hyperfractionated patients had an absolute 8.2% improvement in overall survival at 5 years compared to a 2% absolute benefit with accelerated radiotherapy.59
One of the studies included in the meta-analysis, RTOG 90-03, compared conventional fractionation (70 Gy in 2-Gy daily fractions) to hyperfractionation (81.6 Gy in 1.2 Gy twice daily) to accelerated fractionation with a split course (67.2 Gy in 1.6 Gy twice daily with a 2-week rest after 38.4 Gy) to accelerated fractionation with concomitant boost regimen (72 Gy in 1.8-Gy fractions for 14 fractions followed by a 1.8-Gy morning and a 1.5-Gy afternoon boost to gross disease). Although all primary sites other than the nasopharynx were included, 60% of patients included had oropharyngeal primary tumors. Improved locoregional control was seen in both the hyperfractionated and accelerated concomitant boost arms.63 These improvements resulted in trend toward improved disease-free survival for patients treated with hyperfractionation (37.6% vs. 31.7%, p = 0.067) and accelerated concomitant boost (39.3% vs. 31.7%, p = 0.054), which almost reached statistical significance at the p = 0.05 level. These improvements were associated with an increase in both acute and late toxicity in all three accelerated treatment arms. No significant difference in overall survival was seen.
Simultaneous Integrated Boost Radiotherapy
With the increasing use of intensity-modulated radiotherapy (IMRT), simultaneous integrated boost radiotherapy has been investigated for oropharyngeal cancer patients. The RTOG completed a study (00-22) in early-stage (T1-2, N0-2) oropharyngeal cancer patients treated with bilateral neck radiotherapy54 using doses of 2.2 Gy, 2 Gy, and 1.8 Gy to gross tumor, intermediate-risk, and low-risk planning target volumes (PTVs), respectively. The 2-year risk of local progression was 9% and was higher in patients who had significant underdosing of known tumor. Additionally, no local recurrences, distant metastases, or second cancers were seen in never smokers, possibly representing a surrogate for HPV-related disease, compared to seven locoregional recurrences, five second cancers, and one case of distant metastases in smokers. Two-year overall survival was 95%, and disease-free survival was 82%. Therefore, it appears that for patients with early-stage oropharyngeal cancer treated with radiotherapy alone, simultaneous integrated boost radiotherapy is a viable treatment option.
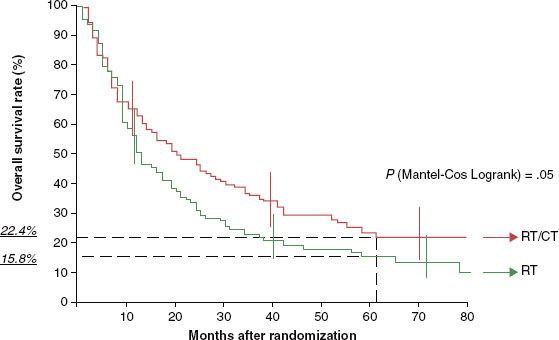
FIGURE 45.4. Overall survival among patients with oropharyngeal cancer treated with radiotherapy alone (RT) or with radiotherapy with concomitant chemotherapy (RT/CT) as analyzed by the Kaplan-Meier method on GORTEC 94-01. (From Denis F, Garaud P, Bardet E, et al. Final results of the 94-01 French Head and Neck Oncology and Radiotherapy Group randomized trial comparing radiotherapy alone with concomitant radiochemotherapy in advanced-stage oropharynx carcinoma. J Clin Oncol 2004;22[1]:69–76. Reprinted with permission. © 2004 American Society of Clinical Oncology.)