Oral Cavity
The oral cavity consists of the lips, oral tongue, floor of the mouth, retromolar trigone, alveolar ridge, buccal mucosa, and hard palate (Figs. 44.1 to 44.3). Classification of tumors by subsite is useful because patterns of spread and clinical outcomes vary by specific subsite, partly reflecting the variable risk of nodal spread by anatomic site of presentation. Cancer of the oral cavity makes up approximately 30% of head and neck region tumors and 3% of all cancers in the United States.1 Surveillance Epidemiology and End Results (SEER) program data estimate 23,880 cases of cancer of the oral tongue, mouth, and oral cavity for 2010 in the United States.2 The incidence rate of oral cancer is more than twice as high in men as in women.3–5 For all stages, the estimated 1-year survival rate after diagnosis is 84%, while the 5-year and 10-year survival rates are 61% and 51%, respectively.3,6 The incidence has been declining by more than 1.4% per year in men and by 1.1% in women since 1992.6 According to American Cancer Society statistics, mortality rates from carcinoma of the oral cavity and pharynx have decreased by 2% per year over the past three decades.4
Worldwide, the incidence of oral cancer parallels the tobacco epidemic. Global estimates suggest 263,900 new cases of oral cancer and 128,000 deaths related to oral cancer in 2008.7 International Agency for Research on Cancer data indicate that the highest rates of oral cancer are found in Melanesia, South-Central Asia, and Eastern Europe. Over the past two decades, oral cancer mortality rates appear to be decreasing in most countries. However, mortality rates in several Eastern European countries, including Hungary and Slovakia, continue to increase.2,8 This unfavorable trend may be associated with the increase of tobacco consumption in women in several countries.8
 ANATOMY
ANATOMY
The anterior boundary of the oral cavity is the skin–vermilion junction. The superior portion of the oral cavity extends posteriorly to the junction between the hard and soft palate, while the inferior portion extends to the circumvallate papillae. The specific anatomic subsites of this region are listed in the following sections.
Lip
The lips begin at the junction of the vermilion border with the skin and form the anterior aspect of the oral vestibule. The lips are composed of the vermilion surface, which is the portion of the lip that comes in contact with the opposing lip. The lips are well defined into an upper and lower. The primary motor control of the lips is provided by the buccal and mandibular branches of the facial nerve.
Oral Tongue
The anterior two-thirds of the tongue is mobile and considered part of the oral cavity. The oral tongue extends anteriorly from the circumvallate papillae to the undersurface of the tongue at the junction of the floor of the mouth. The fibrous septum divides the tongue into right and left halves. The oral tongue can be demarcated into four anatomic areas: the tip, lateral borders, dorsal surface, and undersurface (ventral surface). There are six pairs of muscles that form the oral tongue. Three of these muscles are extrinsic, while the other three are intrinsic. The extrinsic muscles include the genioglossus, hyoglossus, and styloglossus. The intrinsic muscles include the lingual, vertical, and transverse muscles. The former primarily move the body of the tongue, while the latter alter the shape and conformation of the tongue during speech and swallowing. The blood supply to the tongue is primarily via the lingual artery, tonsillar branch of the facial artery, and ascending pharyngeal artery with primary drainage by the internal jugular vein. General sensation of the anterior two-thirds of the tongue is supplied by the lingual nerve. Excluding the circumvallate papillae, taste fibers from the anterior two-thirds of the tongue run in the chorda tympani branch of the facial nerve; the glossopharyngeal nerve provides sensation and taste to the posterior third of the tongue and circumvallate papillae.
FIGURE 44.1. A: Oral cavity surface anatomy. B: Floor of mouth surface anatomy. (From Putz and Pabst. Sobotta atlas of human anatomy, 14th ed. © 2008, Elsevier GmbH, Urban & Fischer, Munich, with permission.)

Floor of the Mouth
The floor of the mouth is a semilunar space extending from the lower alveolar ridge to the undersurface of the tongue. The floor of the mouth overlies the mylohyoid and hyoglossus muscles. The posterior boundary of the floor of the mouth is the base of the anterior tonsillar pillar. This region is divided into right and left by the frenulum of the tongue and contains the ostia of the submandibular and sublingual salivary glands. A sling formed by the mylohyoid muscles medially supports the anterior floor of the mouth, and the hyoglossus supports the posterior floor of the mouth. The lingual and hypoglossal nerves are lateral to the hyoglossus, while the lingual artery is medial to the hyoglossus. Innervation of the floor of the mouth is provided by the lingual nerve.
FIGURE 44.2. Oral cavity; paramedian section depicting regional anatomy. (From Putz and Pabst. Sobotta atlas of human anatomy, 14th ed. © 2008, Elsevier GmbH, Urban & Fischer, Munich, with permission.)

Hard Palate
The hard palate extends from the inner surface of the superior alveolar ridge to the posterior edge of the palatine bone. This is a semilunar area between the superior alveolar ridge and the mucous membrane covering the palatine process of the maxillary palatine bones.
Alveolar Ridge
The alveolar ridges include the alveolar processes of the maxilla and mandible and the overlying mucosa. The mucosal covering of the lower alveolar ridge extends from the line of attachment of mucosa in the buccal gutter to the line of free mucosa of the floor of the mouth. The lower alveolar ridge extends to the ascending ramus of the mandible posteriorly. The superior alveolar ridge mucosa extends from the line of attachment of mucosa in the upper gingival buccal gutter to the junction of the hard palate. The posterior margin is the upper end of the pterygopalatine arch.
Retromolar Trigone
The retromolar trigone is the triangular area overlying the ascending ramus of the mandible. The base of the triangle is formed by the posteriormost molar, and the apex lies at the maxillary tuberosity.
Buccal Mucosa
The buccal mucosa includes the mucosal surfaces of the cheek and lips from the line of contact of the opposing lips to the pterygomandibular raphe posteriorly. This extends to the line of attachment of the mucosa of the upper and lower alveolar ridge superiorly and inferiorly. Innervation is supplied by the buccal nerve, a branch of the mandibular nerve.
 EPIDEMIOLOGY
EPIDEMIOLOGY
The epidemiology of oral cancer strongly reflects exposure to certain environmental agents, particularly tobacco and alcohol. Worldwide, the incidence of oral cancer varies considerably. The International Agency for Research on Cancer notes that the age-standardized incidence rate of oral cavity cancer in economically developed areas in 2008 was 6.9 per 100,000; the age-adjusted rate in economically developing areas was 4.6 per 100,000.7 In Western Europe and the United States the age-adjusted incidence of oral cavity cancer is approximately 7.0 per 100,000 in men, and about half that number in women.7 The incidence of oral cancer in both men and women is highest in Melanesia where the rate is 24.0 per 100,000 in men and 12.0 per 100,000 in women.7 In Central Asia and Eastern Europe the incidence of oral cancer in men is also among the highest in the world: 9.4 and 9.1 per 100,000, respectively. The lowest incidence of oral cancer is found in Middle Africa and Eastern Asia.7
There is a strong causal relationship between smoking and cancer of the oral cavity. Smoking is identified as an independent risk factor in 80% to 90% of patients.9–11 Tobacco users have a fivefold to 25-fold higher risk of oral cavity and oropharyngeal cancer.12 Cessation of smoking is associated with a decline in the risk of cancer of the oral cavity. Abstaining from the use of cigarettes results in a 30% reduction in the risk of cancer in those who quit after 1 to 9 years; the risk is reduced by 50% in those who quit for more than 9 years.11 In India the habit of chewing betel nut leaves rolled with lime and tobacco (mixture known as “pan”), which results in prolonged carcinogen exposure to the oral mucosa, is thought to be the leading cause of oral cancer.13,14 The practice of “reverse smoking” (smoking with the lighted end of the cigar in the mouth, also known as Chutta), peculiar to certain parts of India, is associated with an increase in cancer of the hard palate.15 The combined use of alcohol and tobacco may have a synergistic effect on carcinogenesis.12 International Head and Neck Epidemiology Consortium (INHANCE) pooled analysis data demonstrate a greater than multiplicative joint effect between tobacco and alcohol on head and neck cancer risk, which is most pronounced in pharyngeal and oral cavity cancer.16 Data from a large Japanese cohort study suggest that male smokers have a 2.6 relative risk of death from oral and pharyngeal cancer compared to nonsmokers; the relative risk of death for smoking and drinking combined is 3.3.17
The oral cavity is the most common site for head and neck cancer in the United States.18 Carcinoma of the oral cavity commonly afflicts patients in the sixth to seventh decades of life.5,18,19 Recent SEER data indicate that the age-standardized incidence rate (ASR per 100,000 persons) of oral cavity and pharyngeal cancer in the United States is 15.7 in men and 6.2 in women.3 Although the incidence rates of oral and pharyngeal cancer between Caucasians and African Americans are similar, the mortality rate for African American males is 6.3/100,000, nearly double that of Caucasian males (3.1/100,000).3
FIGURE 44.3. Oral cavity illustration depicting regional anatomy. (From Putz and Pabst. Sobotta atlas of human anatomy, 14th ed. © 2008, Elsevier GmbH, Urban & Fischer, Munich, with permission.)
Although there has been a declining trend in the overall incidence of oral cavity squamous cell carcinoma over the past 30 years, recent studies suggest the incidence of this disease in young adults may be on the rise worldwide.5,19 SEER data suggest that 11.3% of oral cancer cases occur in patients under the age of 45.19 Institutional series suggest that 4% to 6% of oral cancers now occur at ages younger than 45 years.19,20 A recent study by Patel et al.5 suggests that this increasing trend is most pronounced in young white women.5 Reports examining risk factors for oral cancer in the young provide evidence that many younger patients have never smoked or consumed alcohol; predisposition to genetic instability has been hypothesized as a causative factor. Early published series suggest that young patients with oral cancer have a worse prognosis.19 However, recent matched-control studies and national database reviews suggest that outcomes in this population of patients are probably similar to that of older patients.5,19
Ultraviolet radiation has been associated with carcinoma of the lip. In geographic regions where there are long daily periods of sun exposure, cancer of the lip may represent up to 60% of all cancers of the oral cavity.21 Herpes simplex virus (HSV) and human papilloma virus (HPV) have also been implicated in the etiology of oral cavity cancer. The former has been shown to act as a cocarcinogen with tobacco and ultraviolet light in animal models.22,23 The relationship between HPV and oropharyngeal cancer has been well established,24,25 but the association with oral cavity cancer is less clear. Approximately 50% of patients with oropharyngeal cancer and 0% to 20% with oral cavity cancer are positive for HPV 16 DNA.5 A meta-analysis of 17 studies demonstrated a weak association between HPV and oral cancer.26
Certain syndromes such as Plummer-Vinson (characterized by iron-deficiency anemia, hypopharyngeal webs, weight loss, and dysphagia) have been associated with oral cavity cancer. However, Plummer-Vinson syndrome is rare and accounts for a small number of cancers of the oral cavity. Disorders such as xeroderma pigmentosum, ataxia telangiectasia, Bloom syndrome, and Fanconi’s anemia are a result of defective “caretaker” genes. Because such defects result in genetic instability, an increased incidence of second primary malignancies has been reported in this population.27 For instance, an aggressive form of early adulthood head and neck carcinomas, including oral cancer, is seen in patients with Fanconi’s anemia.19 By contrast, with the exception of Li Fraumeni syndrome, abnormalities in “gatekeeper” genes, which inhibit cell proliferation and/or promote cell death, do not appear to predispose to oral cancer. However, despite such reports, the genetics of oral cavity cancer have not been well delineated.28
In patients with cancer of the oral cavity the risk of developing a second primary cancer is well recognized. The concept of field cancerization described by Slaughter and Smejkal29 in 1953 and Day et al.30 may explain the significantly higher rate of second malignancy in patients with head and neck cancers compared to the general population. In an analysis of 851 patients with squamous cell carcinoma of the head and neck, 19% of the study population developed a secondary head and neck carcinoma 5 years after undergoing initial therapy.31 The probability of developing a second metachronous malignancy at 5 years was 22% (18% for the subset of patients with oral cavity cancer).31 Day and Blot32 evaluated the risks of subsequent malignancies in 21,371 patients with oral and pharyngeal cancers between 1973 and 1987. The rate of development of second tumors was 3.7% per year. The risk of second primary cancer was 2.8 times greater than expected, with a 20-fold increase of oral or esophageal cancers and a fourfold to sevenfold increase of respiratory cancers. In a meta-analysis patients with carcinoma of the oral cavity had the highest rate of second primary cancers, most of which occurred in the upper aerodigestive tract.33 An analysis of results from a large intergroup randomized chemoprevention study demonstrated that the incidence of second primary cancers is highly influenced by smoking.34 Second primary cancers have an adverse effect on prognosis and are the major cause of treatment failure in patients with early-stage disease.31,35
 MOLECULAR BIOLOGY
MOLECULAR BIOLOGY
In parallel to the Fearon and Vogelstein model describing the genetic basis of colon cancer,36 there are a series of specific genetic events that precede the development of oral squamous cell carcinoma.37,38 Cancer progression models describe several steps that occur during tumor development: oncogenes become activated and tumor-suppressor genes become deactivated and a series of these alterations are required for carcinogenesis. In the oral mucosa this genetic progression is reflected histologically by the transformation from normal mucosa to dysplastic epithelium and ultimately to frankly invasive squamous cell carcinoma. Data to support this model come from studies that reveal genetic alterations in histologically normal tissues and in premalignant lesions, including loss of heterozygosity at chromosomes 3p14 and 9p21. Furthermore, mutations in the region of chromosome 17p13, which encompasses the tumor-suppressor gene TP53, are among early events that contribute to malignant transformation. Indeed, biopsies of normal mucosa from patients with upper aerodigestive tract carcinomas frequently harbor TP53 mutations.
Additional mutations have been observed in known cancer-related genes encoding proteins such as p16, H-ras, phosphatidylinositol-3-kinase (PI3 K), and F-box/WD repeat-containing protein 7 isoform 1 (FBXW7). Recent whole exome sequencing studies indicate that inactivating mutations in Notch1 are found in close to 15% of tumors evaluated.39 This gene is primarily associated with cellular development and differentiation and encodes a transmembrane receptor with an extracellular domain containing numerous EGF-like repeats. Upon ligand activation, Notch1’s intracellular portion is cleaved and translocated to the nucleus where it can induce genes that are both prosurvival and differentiation as well as antiproliferative. This duality appears to be cell type specific, leading to the observation of Notch1 being either tumorigenic or a tumor suppressor depending on the cancer type. The precise role of Notch1 inactivation in oral squamous carcinoma is currently under investigation; but structural abnormalities in the Notch1 gene found in oral squamous carcinoma specimens suggest that Notch1 functions more like a tumor suppressor in this tumor type.
The aforementioned mutations contribute to changes in critical cellular processes that regulate growth, survival, immortality, tissue invasion, and new blood vessel formation that subsequently lead to changes in the biology of epithelial cells whereby they acquire distinct histologic characteristics. How the same environmental exposures lead to tumor development in some individuals and not others may be explained in part by genetic susceptibility to tobacco carcinogens.40 This susceptibility has been linked to the ability to repair DNA damage and/or metabolize tobacco-related carcinogens. Adding to the complexity of carcinogenesis in this setting is the possibility that alterations in multiple enzymes participating in carcinogen metabolism may be required to exert a sensitizing effect, thus confounding efforts to establish a relationship to any one specific alteration.41
 NATURAL HISTORY AND PATTERNS OF SPREAD
NATURAL HISTORY AND PATTERNS OF SPREAD
Premalignant Lesions
Leukoplakia
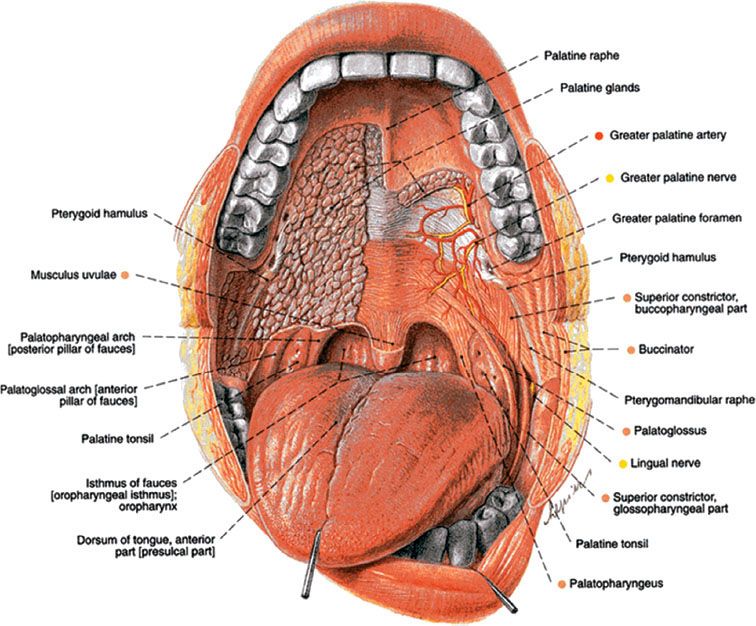
Leukoplakia and erythroplakia are gross clinical descriptors that do not always correspond directly to specific pathologic entities.28,42 The World Health Organization defines leukoplakia as a white patch or plaque that cannot be rubbed off or characterized clinically or pathologically as any other disease42 (Fig. 44.4). Leukoplakia is not related to the presence or absence of dysplasia; however, it is the most common precursor of cancer of the oral cavity. Leukoplakia has a varied clinical appearance, and its appearance frequently changes over time. This is primarily a clinical entity, with certain key pathologic features. These features include hyperkeratosis and acanthosis. Leukoplakias begin as thin gray or gray/white plaques that may appear somewhat translucent, are sometimes fissured or wrinkled, and are typically soft and flat. They frequently have sharply demarcated borders but occasionally blend gradually into normal surrounding mucosa.
Homogenous leukoplakia is a uniform white lesion that is prevalent in the buccal mucosa. These lesions represent the most common variety of leukoplakia and have a low malignant potential. Conversely, high-risk oral leukoplakia demonstrates abnormal orientation of cells, nuclear hyperchromatism, increased mitosis, and a nuclear cytoplasmic ratio.28 Clinically these lesions are nonhomogenous, nodular, speckled, or verrucous, with central ulceration or erosion.42,43 Follow-up studies demonstrate that between <1% and 18% of oral leukoplakias develop into oral cancer, with the latter clinical subtype conferring a higher risk of malignant transformation.44,45
The natural history of leukoplakia is variable. Leukoplakia may regress spontaneously without therapy. A baseline biopsy can be performed to establish diagnosis and rule out malignant transformation. Leukoplakia with clinically or histologically aggressive features, demonstrating dysplasia, should be excised.
Erythroplakia
The term erythroplakia describes a chronic, red, generally asymptomatic lesion or patch on the mucosal surface that cannot be attributed to a traumatic, vascular, or inflammatory cause. Erythroplakia, like leukoplakia, is a clinical diagnosis of exclusion that requires the clinician to rule out all other erythematous oral lesions.46 However, erythroplakia is associated with a higher risk of malignant transformation than leukoplakia. Transformation rates are considered to be the highest among all precancerous oral lesions and conditions.47 Histopathologically it has been documented that in homogenous oral erythroplakia, 51% showed invasive carcinoma, 40% carcinoma in situ, and 9% mild or moderate dysplasia.47 The treatment of choice for erythroplakia is surgical excision.
Oral Submucous Fibrosis
The term describes a generalized fibrosis of the oral cavity tissues resulting in marked rigidity and trismus. At early stages these premalignant lesions are characterized by blanching of the mucosa with a marble-like appearance. At more advanced stages, palpable fibrous bands become evident around the buccal mucosa and the mouth opening. Once oral submucous fibrosis reaches advanced stages, approximately 25% of cases biopsied demonstrate epithelial dysplasia in addition to subepithelial alterations.12 Oral submucous fibrosis is associated with the use of betel quid (with or without tobacco) or pan masala.12 In India, it is estimated that as many as 5 million individuals are afflicted with oral submucous fibrosis.12
FIGURE 44.4. A: Superficial patches of leukoplakia involving the lateral and ventral surfaces of the oral tongue. B: Extensive leukoplakia involving the ventral oral tongue, floor of the mouth, and mandibular alveolus.
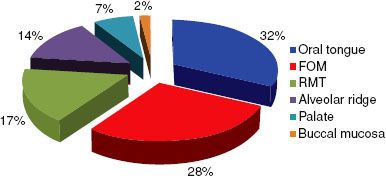
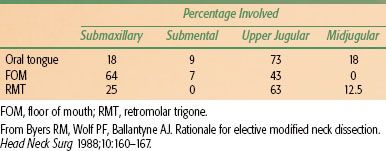
FIGURE 44.5. Subsite distribution of 3,308 de novo cancers of the oral cavity treated at the University of Texas M.D. Anderson Cancer Center from 1970 to 1999. FOM, floor of mouth; RMT, retromolar trigone. (Adapted from Chen AY, Myers JN. Pathogenesis and progression of squamous cell carcinoma of the oral cavity. Dis Mon 2001;47:275–361.)
Oral Cavity Cancer
Relative Distribution
The most common subsite for squamous cell carcinoma of the oral cavity (excluding the lip) is the oral tongue (Fig. 44.5). In a review of 3,308 cases of oral cavity cancer treated at the University of Texas M.D. Anderson Cancer Center between 1970 and 1999, 32% involved the oral tongue.28 The floor of the mouth is the second most common subsite where oral cavity carcinomas may arise. Carcinoma of the alveolar ridge accounts for approximately 10% of oral cavity carcinomas. Squamous cell carcinoma of the retromolar trigone and hard palate is rare. Similarly, carcinoma of the buccal mucosa is rare in the United States but is the most common carcinoma of the oral cavity in Southeast Asia because of the widespread use of betel nut.28
Patterns of Spread
Local Spread
Carcinoma from distinct anatomic subsites may exhibit different tendencies for spread based on natural anatomic barriers and location. For instance, the majority of lip cancers are local growths that do not invade deeply into the tissues of the oral cavity or mandible.48 However, a select few lip carcinomas may be deeply invasive with perineural involvement, posterior spread to involve cortical bone, extension to the inferior alveolar nerve, or spread to the skin of the face (Fig. 44.6). Squamous cell carcinoma of the floor of the mouth can secondarily involve the ventral tongue, extend along the lingual nerve or submandibular duct, or invade the cortex of the mandible. Tumors in this location can invade deeply, involving the muscles of the floor of the mouth. There is an anatomic gap between the mylohyoid and hyoglossus muscles through which a carcinoma can gain access to submandibular and sublingual areas. Carcinomas of the alveolar ridge and retromolar trigone tend to invade bone early. Tumors of the inferior alveolar ridge may access the mandibular canal and the inferior alveolar nerve, while tumors of the superior alveolar ridge may pass into the maxillary antrum or floor of the nose. Infiltrating lesions of the buccal mucosa can invade the buccinator muscle, extend to the buccal fat pad, and invade the subcutaneous tissue. The hard palate has a relatively dense mucoperiosteum that is relatively resistant to tumor invasion. However, the primary and secondary palates are fused at the incisive fossa, where tumors can gain access into the nasal cavity. The greater palatine foramina can allow tumors to spread posteriorly and enter the pterygopalatine fossa and skull base.
FIGURE 44.6. Advanced, destructive, ulcerative squamous cell carcinoma involving the lower lip, buccogingival soft tissues, and skin in a patient with long-term habit of tobacco chewing.
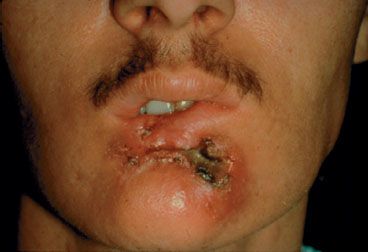
TABLE 44.1 RELATIVE INVOLVEMENT OF LYMPH NODE REGIONS BY ORAL CAVITY SUBSITE
Lymphatic Metastases
For the purpose of staging and treatment planning, the neck is generally divided into five primary levels. Level I includes the submental (Ia) and submandibular (Ib) triangles. Level II includes the upper jugular chain lymph nodes from the base of the skull to the carotid bifurcation and from the sternohyoid muscle anteriorly to the posterior border of the sternocleidomastoid posteriorly. Level III includes the midjugular nodes, which extend from the carotid bifurcation to the omohyoid muscle inferiorly, the sternohyoid medially, and the posterior aspect of the sternocleidomastoid posteriorly. Level IV includes the inferior jugular nodes, bounded by the omohyoid muscle superiorly, the clavicle inferiorly, and the posterior aspect of the sternocleidomastoid posteriorly. Level V includes nodes in the posterior triangle, bordered by the base of the skull superiorly, clavicle inferiorly, and posterior aspect of the sternocleidomastoid anteriorly.
The oral cavity has an extensive group of lymphatics that manifest a fairly predictable lymph node drainage pattern based on location (subsite) within the oral cavity49 (Table 44.1). The upper and lower lip demonstrates distinct patterns of lymphatic drainage. The principal lymphatic drainage of the upper lip is to preauricular, periparotid, submental, and submandibular lymph nodes, which secondarily drain to deep jugular lymph nodes. The medial portion of the lower lip drains primarily to the submental lymph nodes, while the lateral portion drains to the submandibular triangle.
A classical study by Lindberg50 demonstrated that the superior deep jugular nodes are most frequently involved by cancers of the oral cavity. The oral tongue has an extensive lymphatic drainage. The anterior portion of the tongue drains to the submental nodes (level Ia), and the lateral portion drains to the submandibular (level Ib) and deep jugular nodes (level II). The posterior oral tongue drains into the upper jugulodigastric group of lymph nodes (level II). The lymphatics of the oral tongue also have extensive communication across the midline; thus, carcinomas of the oral tongue can metastasize bilaterally. Studies suggest that some carcinomas of the lateral oral tongue may metastasize to level IV lymph nodes without involving levels I, II, or III.51 This implies that there may be separate lymphatic channels draining from the oral tongue directly to level IV nodes, allowing for apparent “skip metastases.”
Dye injection studies have shown that the floor of the mouth has superficial and deep lymphatic drainage systems.51 The superficial system crosses randomly in the midline and drains into both the ipsilateral and contralateral submandibular lymph nodes. The deep lymphatic system is thought to penetrate the periosteum and drains into the submandibular and upper jugular lymph nodes. Lymphatics from the buccal mucosa drain into the periparotid, submental, and submandibular nodes. Tumors of the alveolar ridge may drain into the submental and submandibular triangles, upper deep jugular, and retropharyngeal lymph nodes. Tumors of the inferior alveolus are more likely to metastasize to the neck than tumors of the superior alveolus. The main lymphatic drainage from the retromolar trigone is into the superior-deep jugular lymph nodes; however, there may be some drainage into periparotid and retropharyngeal lymph nodes. Lymphatics in the hard palate are few, but drainage is into submandibular, superior deep jugular, and retropharyngeal nodes.
The risk of neck metastases depends on several factors including site and size of the primary tumor. Overall, for patients with squamous cell carcinoma of the oral cavity, cervical metastases occur in approximately 30% of cases.43 The rate of neck metastases for carcinoma of the lip is approximately 10%.43 Squamous cell cancer of the oral tongue carries the highest risk of nodal metastases. The frequency of neck metastases can range from 15% to 75%, depending on the size of the primary lesion.50,52 Approximately 25% of patients with carcinoma of the oral cavity will have occult nodal metastases, and 3% of patients will have contralateral metastases.50,52 Contralateral metastases are more common in tumors that approach or cross the midline. Early tumors of the floor of the mouth have approximately a 12% to 30% incidence of occult nodal metastases depending on the thickness of the lesion, while larger lesions can have an incidence of nearly 50%.53 Approximately 15% to 20% of upper alveolar ridge tumors will involve the neck at presentation; the risk of occult metastases in a clinically negative neck is approximately 15% to 20%.50 The incidence of neck metastases in lower alveolar ridge tumors is higher than for tumors of the upper alveolar ridge.50 For cancers of the buccal mucosa, the incidence of positive cervical lymph nodes at diagnosis is 10% to 30%; the incidence of pathologically positive nodes in a clinically negative neck is about 15%. Similar rates of occult metastases occur for squamous cell carcinoma of the retromolar trigone; however, patients tend to present with more advanced disease, resulting in a somewhat higher rate of regional metastases.43 The incidence of lymph node involvement from carcinoma of the hard palate is low, approximately 15%.48,54
Distant Metastases
The majority of oral cavity cancers present as localized disease and remain localized until late in the course of their development. Distant metastasis occurs in approximately 15% to 20% of patients who eventually die of their disease.43 The risk of distant metastasis increases with the degree of lymph node involvement. Patients with recurrent disease are also at higher risk for distant metastases.55 Patients without clinically appreciable neck disease rarely fail distantly after treatment. In general terms with respect to head and neck cancer, 66% of distant metastases are to the lungs, 22% to the bones, and 9.5% to the liver.56 On rare occasion, the oral cavity will serve as a site for distant metastasis from another anatomic primary tumor site (Fig. 44.7).
FIGURE 44.7. Unusual oral cavity metastasis in a patient with known renal cell carcinoma. This advanced, hemorrhagic metastasis showed identical pathology to the patient’s known renal cell carcinoma.
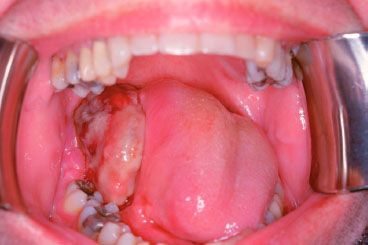
FIGURE 44.8. Kaposi’s sarcoma involving the hard palate and maxillary alveolus in a patient with human immunodeficiency virus.
 PATHOLOGIC CLASSIFICATION
PATHOLOGIC CLASSIFICATION
The predominant histopathologic type of cancer in the oral cavity is squamous cell carcinoma. There are several variants of squamous cell carcinoma, including basaloid and verrucous carcinoma. Basaloid squamous cell carcinoma is believed to have a worse prognosis than traditional squamous cell carcinoma. In a retrospective comparison between basaloid squamous cell carcinoma and traditional poorly differentiated squamous cell carcinoma, the former had a higher incidence of advanced disease at presentation, distant metastases, and poorer overall survival rate.57 Verrucous carcinoma is a less common variant of squamous cell carcinoma. It is generally considered a low-grade malignancy with low metastatic potential and good overall prognosis, although often with challenges for local control in elderly patients.28 For most cases, adjuvant radiation and elective neck dissection are not indicated. Sarcomatoid carcinomas can be found in the oral cavity and larynx. This variant of squamous cell carcinoma carries a poor prognosis with a mean survival of approximately 2 years.58
Less than 10% of neoplasms of the oral cavity have nonsquamous histology. Most of these are minor salivary gland tumors, which tend to arise in the hard palate. Adenoid cystic carcinoma accounts for approximately 30% to 40% of minor salivary gland cancers of the oral cavity.59 Other histologies that can occur in the oral cavity include adenocarcinomas, melanoma, ameloblastoma, lymphoma, and Kaposi’s sarcoma (Fig. 44.8). Approximately 50% of acquired immunodeficiency syndrome–related cases of Kaposi’s sarcoma have oral cavity involvement.43 Most lymphomas in the head and neck arise in Waldeyer’s ring (tonsil, base of tongue, and nasopharynx). Only 2% of all lymphomas are found in the oral cavity.60 Fortunately, melanoma of the oral cavity is very rare and represents only 0.2% to 8% of all melanomas.61 Mucosal melanomas generally have a worse prognosis than cutaneous melanomas.
 CLINICAL PRESENTATION
CLINICAL PRESENTATION
The oral cavity is an anatomic region that is readily accessible to visual inspection and palpation. Despite this fact, many patients with oral cavity tumors present with advanced-stage disease as initial symptoms may be vague and painless. Tumors of the oral tongue often present as small ulcers and gradually invade the musculature of the tongue. Advanced lesions may be either ulcerative or exophytic and are usually quite evident. Some cancers of the oral tongue are painful even in their early stages. Cervical metastases occur early in the natural history of the disease, with 30% to 40% of patients harboring cervical lymph node metastases at diagnosis. Squamous cell carcinomas of the oral tongue most often arise along the lateral borders of the tongue28 (Fig. 44.9).
Lesions of the floor of the mouth are often infiltrative and may invade bone, the muscles of the floor of the mouth, and the tongue. The frenulum is frequently a site of involvement. Clinical fixation of the tumor to the mandible suggests periosteal involvement, which may occur early.
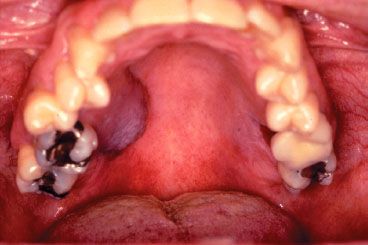
Tumors of the alveolar ridge may present with pain while chewing, loose teeth, or ill-fitting dentures in edentulous patients. These cancers often arise in edentulous areas or along the free margin of the mandibular alveolus (Figs. 44.10 and 44.11). Anesthesia of the lower lip and teeth may indicate involvement of the mandibular canal and inferior alveolar nerve.
Tumors involving the retromolar trigone region may present with an exophytic growth pattern and limited involvement of underlying bone (Fig. 44.12), or they may infiltrate cortical bone and spread along regional tissue planes to involve the pterygoid complex and parapharyngeal space. These latter lesions often induce trismus early in the clinical course.
Carcinoma of the buccal mucosa is rarely symptomatic early in its course. Lesions may be papillary or erosive and located near the dental occlusal line. These tumors are often relatively asymptotic and therefore seldom come to medical attention as T1 lesions. Often, these tumors manifest associated leukoplakia. Multiple primary sites and local recurrence are also common. These tumors most frequently arise adjacent to the lower molars along the occlusal line of the teeth.
Carcinoma of the hard palate is often painless, and the sole presenting symptom may be an irregularity in the mucosa or ill-fitting dentures. Other presenting symptoms include nonhealing ulcers of the hard palate, intermittent bleeding, and pain.
FIGURE 44.9. T2N0M0 squamous cell carcinoma involving the right lateral oral tongue.
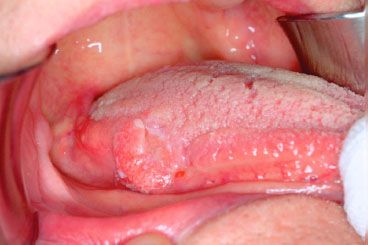
FIGURE 44.10. T1N0M0 squamous cell carcinoma of the mandibular alveolus. No evidence of bone invasion identified on Panorex or computed tomography imaging.
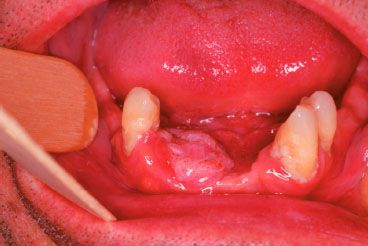
FIGURE 44.11. Squamous cell carcinoma involving the mandibular alveolus and buccogingival space.
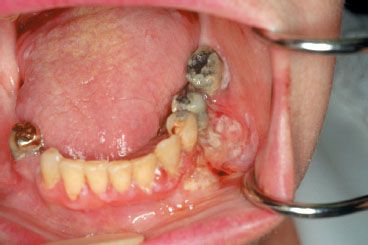
FIGURE 44.12. Exophytic T3 carcinoma involving the right retromolar trigone, anterior tonsillar pillar, and proximal soft palate with minimal infiltration into the right base of the tongue.

 DIAGNOSTIC EVALUATION
DIAGNOSTIC EVALUATION
Patients with oral cavity cancer should undergo a comprehensive history and physical examination. Detailed examination is particularly important for oral cavity tumors in that much can be learned about cancers that afford opportunity for direct visual inspection and digital palpation. A biopsy of lesions in question should be obtained as well as a thorough dental assessment. Computed tomography (CT) scans, panoramic radiographs, magnetic resonance imaging (MRI), and other imaging studies may also be important for accurate staging of the tumor and in treatment planning.
The history of present illness should address the following issues: tobacco and alcohol use; dysphagia; odynophagia; pain; trismus; difficulties with speech; hoarseness; loose teeth; ill-fitting dentures; hypoesthesia of the face, lips, or mandible; weight loss; and malnutrition. Otalgia suggests involvement of the ninth or 10th cranial nerve; facial numbness may suggest involvement of the fifth cranial nerve. Hypoesthesia usually results from perineural invasion, often from penetration of the mandible and perineural spread along the inferior alveolar nerve. The presence of trismus may indicate extension into the pterygoid musculature, signifying locally advanced disease. Other symptoms include a persistent ulcer, bleeding, drooling, or respiratory distress. A patient’s comorbid illnesses must also be taken into account in the treatment plan.
A detailed examination of the head and neck should be performed, with particular focus on the oral cavity and oropharynx. This usually begins with a full inspection of the oral cavity, including a thorough inspection of the teeth. Palpation of the oral cavity can help assess bony involvement, tongue fixation, and depth of involvement. Deviation or fixation of the tongue suggests involvement of extrinsic muscles of the tongue. Bimanual palpation can help assess the depth of tumor invasion into musculature of the tongue and floor of the mouth. A thorough palpation of the neck is important to assess regional nodal disease.
Imaging can complement the physical examination in determining the extent of disease. A chest x-ray should be performed to exclude lung metastases or a second primary cancer. CT is the modality most commonly used to determine the extent of soft-tissue and bony involvement and occult disease in the neck (Fig. 44.13). CT may be used to determine the extent of invasion into the deep musculature of the tongue and adjacent structures. Moreover, CT is a valuable modality for visualizing invasion of the mandible, palate, and pterygopalatine fossa. If CT scanning is not available, then panoramic radiographs can be used to demonstrate mandibular invasion. MRI may be used in case of contrast allergy or a lesion that is not well visualized on CT. For instance, MRI may be used if a patient has significant dental artifact that obscures visualization of the primary tumor on CT. MRI provides excellent definition of tumor involving the tongue and is a good modality for evaluating the possibility of perineural spread. Ultrasound may be used to screen for enlarged lymph nodes that are not clinically detectable. In experienced hands, the accuracy of ultrasound when combined with fine needle aspiration may be superior to CT or MRI for staging the neck.62
Positron emission tomography (PET) and PET/CT have been used increasingly in head and neck cancer evaluation for staging disease in the neck, evaluation of perineural and skull base involvement, identification of distant metastases, and detection of recurrence. Several studies suggest that PET is more sensitive and specific in evaluating lymphatic metastases compared to CT and MRI.63,64 PET/CT may improve the anatomic localization of abnormalities identified on PET and decrease the number of equivocal PET findings.65 Liao et al.66 prospectively evaluated 473 patients with oral cavity squamous cell carcinoma and reported a sensitivity of 77.7% and a specificity of 58% for PET/CT after histopathologic correlation of nodal metastases to the neck. Maddipatla et al.,67 in a prospective study evaluating 552 lymph nodes dissected after oral cancer surgery, reported a 97% negative predictive value (NPV) for PET/CT. Pentenero et al.68 reported an accuracy of 66.7%, a specificity of 76.9%, and an NPV of 83.3% for staging of the neck with PET/CT. Despite the improved overall accuracy, clinical application of PET/CT is limited by the suboptimal detection of small metastases. Therefore, the decision to pursue a neck dissection should not be based solely on PET/CT findings.
Reports have indicated that the overall sensitivity and specificity of PET may be superior to CT and MRI for evaluating persistent or recurrent disease, particularly in patients who have received previous radiotherapy.69,70 The accuracy of PET in detecting disease recurrence or persistence may be influenced by the time posttreatment when images are acquired. It is recommended that a PET scan not be performed until 8 to 12 weeks posttreatment to minimize the risk of both false-negative and false-positive interpretations.71–74
FIGURE 44.13. A: Transverse computed tomography image with contrast depicting infiltrative squamous cell carcinoma of the left lateral oral tongue and floor of the mouth with associated bone destruction. There is posterior tumor extension to involve the retromolar trigone and tonsillar complex. B: Corresponding computed tomography bone window views demonstrating destruction of the mandibular body.
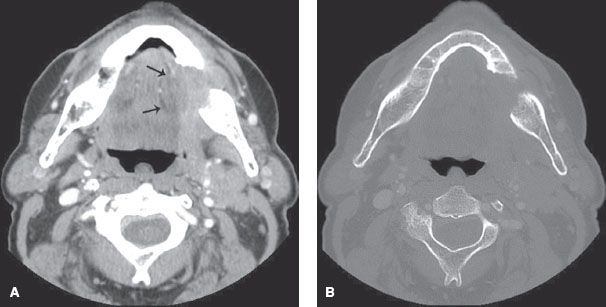
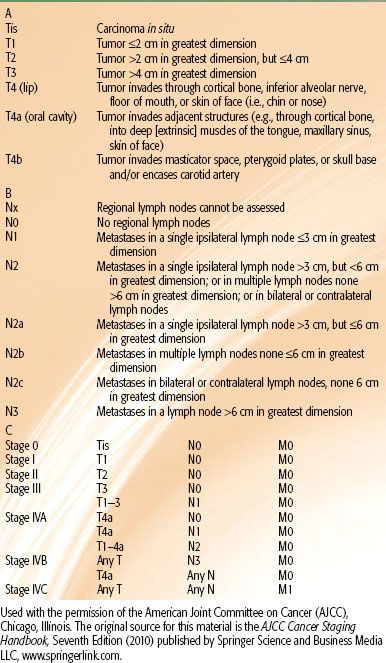
TABLE 44.2 STAGING OF ORAL CAVITY CARCINOMA
 CLINICAL STAGING
CLINICAL STAGING
The American Joint Committee on Cancer has established a staging system for all cancers of the oral cavity (Table 44.2).75 Nonepithelial malignancies and melanoma of the lip and oral cavity are not included.
 GENERAL MANAGEMENT
GENERAL MANAGEMENT
The choice of treatment modality, either singly or in combination, depends on the stage and size of the tumor and relevant patient factors such as toxicity, performance status, comorbid disease, and convenience. The overall health and functional status of the patient are important determinants in choosing between surgical and nonsurgical approaches. A multidisciplinary approach is paramount in the management of oral cancer patients. It is important and valuable for patients to undergo evaluation by relevant members of the multidisciplinary team, including head and neck surgery, radiation and medical oncology, nursing, dentistry, dietary, speech pathology, and social work, before treatment is delivered. Multidisciplinary evaluation prior to treatment disposition helps to ensure that broad consensus treatment recommendations are made and interdisciplinary coordination of care is facilitated.
Surgery is most commonly the treatment of choice. Surgical resection is expeditious, effective, and often associated with modest morbidity and good functional outcome particularly for patients with small to moderate-size lesions. Radiation therapy can be considered for patients with early-stage disease who either are not surgical candidates or refuse surgical management. For patients with advanced lesions of the oral cavity, a combined-modality approach is generally recommended. In patients with high-risk pathologic features, the addition of concurrent chemotherapy during the postoperative radiation treatment course may further augment tumor control rates provided the chemotherapy can be tolerated.76–78 High-risk features commonly include extracapsular tumor spread and positive resection margins.77,79–81
 SURGICAL MANAGEMENT
SURGICAL MANAGEMENT
Cancer of the oral cavity is most commonly treated surgically when the disease is in its early stages.28 Because successful treatment of oral cavity carcinoma relies on effective management of the regional lymphatics as well as the primary cancer, the neck should be addressed in treatment planning. Elective neck surgical treatment is often used for management of the clinically node-negative patient with oral cancer, and therapeutic neck dissections are performed for patients with clinically apparent nodal disease. Postoperative radiation or chemoradiation is administered to those patients with pathologic evidence of extensive nodal disease and/or extracapsular spread. Because of the high occult metastatic rate for many cancers, elective neck treatment is encouraged in all but the earliest stages of primary site disease.
Management of the Oral Cavity
Surgical approaches to cancers of the oral cavity may either be transoral, transcervical (pull-through), or, alternatively, via mandibulectomy, which is sometimes necessary to obtain the exposure required to achieve adequate margins. In cases where the mental or alveolar nerve is involved with tumor, the nerve should be proximally resected and analyzed microscopically. A tracheotomy is often necessary to maintain a patent airway because of the large amount of oral edema resulting from extensive resection and placement of myocutaneous flaps in the oral cavity.
Tumors that approximate the gingiva should be resected with the gingiva and periosteum as an additional deep margin, while those that appear to involve the periosteum should be resected with an additional deep margin of bone. This last procedure is termed a marginal mandibulectomy. Depending on the extent of tumor involvement, this may involve resection of a bicortical rim of bone at the upper aspect of the alveolus (rim mandibulectomy) or, alternatively, selective removal of the inner cortex using a vertical or oblique resection (sagittal mandibulectomy). It is commonly recommended to leave at least a 1-cm-thick segment of bone inferiorly following a rim mandibulectomy to reduce the risk of pathologic fracture. Those lesions that directly invade bone should be resected with a segment of bone. This often requires soft-tissue or osseous reconstruction of the resected bone segment.
Regarding reconstruction after tumor resection, small surgical defects may not require reconstruction and therefore are often allowed to heal by secondary intention. Larger defects may be reconstructed by primary closure, skin graft, regional flap, or free-tissue transfer from different sites. Goals of reconstruction are to replicate the function and appearance of the resected tissue. Urken et al.82 have developed a systematic approach to functional reconstruction of the oral cavity. Their approach to reconstruction is based on the extent and functional status of the residual tongue and the presence or absence of an associated mandibulectomy.
Split-thickness skin grafts are often used for reconstruction and are usually most expedient and efficacious for small defects. Larger defects may require a local or regional flap. Small intraoral defects can be reconstructed effectively with palatal, tongue, and buccal mucosa flaps but usually at the cost of decreased function. Regional flaps that are used in the reconstruction of the oral cavity include the pectoralis major flap, trapezius flap, and latissimus dorsi flap. Continuing developments in microvascular surgery have allowed for head and neck reconstructive surgeons to perform free-tissue transfer to reconstruct oral cavity defects. The free flaps most commonly utilized in the oral cavity are the radial forearm flap, the anterolateral thigh flap, the rectus abdominis flap, and the fibula flap.
Total glossectomy defects are well suited for free flap reconstruction. Reconstruction of the mandible often requires free flaps that contain bone and soft tissue such as the fibula flap, the iliac crest flap, and the scapular flap. Compared to reconstruction plates, free flaps also allow the potential for a sensate flap through neural anastomosis. A sensate flap may result in improved swallowing and speech function, but few studies have unequivocally demonstrated an improvement in these functional outcomes.76,83–87
Management of the Neck
Lymphadenectomy in the presence of known neck disease can be therapeutic as well as provide prognostic information (i.e., presence of extracapsular extension). For midline tumors, regardless of the type of dissection, bilateral surgical management is recommended. However, for well-lateralized tumors, unilateral dissection, followed by bilateral postoperative radiation, is reasonable. Generally, in patients with nodal disease ≥6 cm (N3), extracapsular extension, or clinically evident disease in levels IV or V, the most common approach is a modified radical nodal dissection (MRND). This includes the removal of nodal levels I through V (including the submental nodes), with sparing of the sternocleidomastoid muscle, internal jugular vein, and accessory nerve, if they are uninvolved with disease. However, as MRND can lead to significant morbidity, the use of selective neck dissection has gained favor in patients with more limited nodal disease.88 This procedure involves the removal of at least levels I through III, with the possible addition of level IV depending on the interpretation of the surgeon. Outcomes for selective neck dissection in this patient population compare favorably to those observed with MRND; however, the possibility of skip metastases is always a concern.89
Heretofore, we have discussed the role of nodal dissection in patients with clinically evident nodal disease. However, further neck management questions arise in the patient with a clinically negative neck. The decision to proceed with a selective neck dissection in this context is usually guided by the invasion of the primary oral tumor, with a depth >2 to 4 mm thought to require surgical intervention. Further guidance on this topic is provided by two recent trials investigating the role of sentinel lymph node biopsy (SLNB) in patients with small volume (T1–2) oral squamous cell carcinoma.90,91 Similar to other cancer types, SLNB provides excellent sensitivity (~90% to 100%) and negative predictive value (~95%) with no compromise of local control in the neck. Although a promising approach, SLNB in oral cavity squamous cell carcinoma has yet to become a standard practice in North America.
 RADIATION THERAPY
RADIATION THERAPY
General Principles
Evidence-based practice guidelines in oncology published by the National Comprehensive Cancer Network (NCCN) recommend single-modality treatment (i.e., surgery or radiation) for early-stage T1 or T2 lesions92; however, a primary surgical approach is generally preferred. For more advanced lesions, NCCN guidelines recommend a combined-modality approach involving surgery followed by adjuvant radiation or chemoradiation.92 In considering primary radiation as a treatment option, it must be borne in mind that the keratinizing, well-differentiated histology of many oral cancers may portend radioresistance. It is also important to consider that the bony structures of the mandible and maxilla may impact the transmission of ionizing radiation. A combined approach including both external-beam radiation and interstitial brachytherapy is often recommended for optimal outcome. Thus, a course of radiation can require several weeks of daily therapy followed by an interstitial implant. The normal-tissue toxicity risks of xerostomia, dental and gum injury, and occasional osteoradionecrosis may render radiation therapy a less attractive option for single-modality treatment of patients who are candidates for surgery.
Historically, sites commonly treated with single-modality radiation include the lip, floor of the mouth, and oral tongue.93,94 Early work indicates that the success rate of radiotherapy is higher if some or all of the treatment is administered with brachytherapy.95,96 Decroix and Ghossein97 reported outcomes in 602 patients with cancer of the oral tongue treated with radium implantation or implantation plus external-beam radiation. In this series, recurrence at the primary site or at the primary site and neck was 14% and 22% for T1 and T2 lesions, respectively. The Royal Marsden Hospital reported local control rates of 90% at 5 years for T1 and T2 tumors treated with interstitial radiation with or without external-beam radiation.98 Pernot et al.99 reported local control rates of 96% for T1, 85% for T2, and 64% for T3 lesions of the oral cavity treated with brachytherapy and neck dissection. In this series, locoregional control rates were 83%, 70%, and 44%, respectively. Retrospective studies suggest that control rates at the primary site of early oral cavity lesions treated with brachytherapy alone or a combination of brachytherapy plus external-beam radiation range from approximately 70% to >95%.97–100 For tumors that either involve the mandible or are immediately adjacent to it, definitive radiotherapy is contraindicated because it compromises control and increases the risk of osteoradionecrosis.
Intraoral cone, like interstitial brachytherapy, is a localized radiation therapy technique that has been used to boost the dose to the primary tumor in the oral cavity. Institutions with significant experience with this technique have reported results that rival those obtained by interstitial brachytherapy.101,102 Either technique for boosting the primary tumor has resulted in improved outcomes compared to high-dose radiation therapy alone.103,104 In general, external-beam radiation therapy followed by either technique is preferable over radiation therapy alone. As with all specialized procedures, the skill and experience of the radiation oncologist is of critical importance to the successful delivery and outcome of interstitial radiation or intraoral cone therapy.
The outcomes for advanced lesions of the oral cavity (T3 and T4) are less than satisfactory with either surgery or radiation alone. In most advanced-stage cancers single-modality therapy is inferior to combined-modality therapy.96,105,106 Adjuvant radiation therapy can be delivered preoperatively or postoperatively.101,105 Although each strategy has potential advantages and disadvantages, postoperative radiation therapy is generally preferred. Notable disadvantages of preoperative radiation therapy include a delay in definitive surgical treatment and limitations on the dose of radiation that can be delivered due to the risk of wound complications after surgery. Postoperative radiation treatment carries the advantage of no delay in the implementation of surgical resection and complete pathologic staging of the tumor. However, it must be borne in mind that postoperative wound complications may delay the implementation of postoperative radiation, and the regional hypoxia that can accompany the postoperative state may diminish the effectiveness of radiation compared to that achievable under conditions of full oxygenation.
Adjuvant Radiation
Although surgery is the preferred initial treatment approach for the majority of patients with tumors of the oral cavity, adjuvant radiation is commonly recommended to enhance the likelihood of locoregional tumor control. Robertson et al.107 conducted a phase III study in the United Kingdom of 350 patients with T2–4/N0–2 oral cavity or oropharyngeal cancers comparing surgery and postoperative radiation versus radiation alone. Because a difference in survival was identified, the study was closed early. The authors found that after 23 months, overall survival, cause-specific survival, and local control were all improved in the surgery plus radiation arm. Traditionally, indications for postoperative radiation therapy include multiple cervical metastases, positive or close margins, extracapsular extension, perineural invasion, advanced T stage, and mandibular bone involvement. A phase III study conducted at the University of Texas M.D. Anderson Cancer Center established the relative prognostic significance of clusters of two or more clinicopathologic features.108 The adverse clinicopathologic features in this study included (a) close or positive margins, (b) nerve involvement, (c) two or more positive lymph nodes, (d) largest node >3 cm, (e) treatment delay >6 weeks, and (f) Zubrod performance status ≥2. The presence of extracapsular extension was the only factor independently predictive of locoregional recurrence. Moreover, the authors concluded that escalation of dose beyond 63 Gy (in 1.8 Gy per fraction) to sites of increased risk did not yield improved locoregional control.
It is well appreciated that head and neck tumors are rapidly proliferating. Several retrospective series have demonstrated an association between diminished outcomes and a delay beyond 6 weeks in initiating postoperative radiation.109–111 A multi-institutional prospective study by Ang et al.112 demonstrated that the total treatment time from the completion of surgery to the completion of radiation may affect the likelihood of ultimate disease control. This study illustrated the impact of overall treatment time on 5-year locoregional control: patients with overall treatment times <11 weeks demonstrated a locoregional control rate of 76%, compared to 62% for 11 to 13 weeks, and 38% for times beyond 13 weeks. Hence, it is recommended that adjuvant radiation proceed as soon as surgical wounds are well healed, optimally 4 to 6 weeks after completion of surgery.
There has been significant interest in the use of intensified radiation fractionation schedules to counter rapid tumor cell repopulation as a means of improving outcomes in head and neck cancer patients treated with radiation. Altered fractionation regimens such as hyperfractionation or accelerated fractionation have been considered for patients being treated with radiation alone, as this approach has been demonstrated to improve the likelihood of locoregional tumor control in the definitive setting.113 However, the use of altered fractionation in the postoperative setting has not been resolved. Ang et al.112 reported a trend toward higher locoregional control and survival with a concomitant boost schedule compared to a standard fractionation schedule. However, in a subsequent study, Sanguineti et al.114 demonstrated no benefit for accelerated fractionation, except in patients for whom postoperative radiation was delayed.
There has been recent interest in postoperative chemoradiation for patients with high-risk pathologic features. The results of two randomized trials suggest that postoperative chemoradiation may be beneficial for improving local-regional control and disease-free survival among selected patients with specific high-risk features.77,80 The impact of chemoradiotherapy appears to be most pronounced in patients with extracapsular extension and/or microscopically involved surgical margins.79,80
FIGURE 44.14. Lucite oral cavity mouthpiece fabricated at the University of Wisconsin for patients with oral cavity carcinomas. Upper dentition or maxillary alveolus links to U-shaped notch and tongue rests beneath smooth undersurface of mouthpiece. Note embedded solder wire in mouthpiece floor to facilitate visualization of tongue positioning at the time of simulation and beam design.





