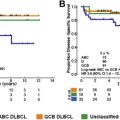
Primary mediastinal B-cell lymphoma is characterized by a high chance of cure, and cured patients have a long disease-free life-expectancy; however, prognosis is severe in the case of relapsed or refractory disease. The initial use of the most effective chemoimmunotherapy regimen is therefore crucial. Understanding who will benefit from postinduction radiotherapy is also of paramount importance; positron emission tomography may be a reliable guide for physicians in determining which patients will require consolidation. New drugs with mechanisms of action including the most relevant biologic features of the tumor may allow better disease control.
Key points
- •
Applying the most effective chemoimmunotherapy regimen will induce the highest rate of complete responses after first-line treatment.
- •
Determining which patients will benefit from consolidation radiotherapy is a key strategy to optimize outcomes.
- •
Minimization of long-term treatment-related toxic effects is necessary, as most patients are likely to be cured and will enjoy long periods free of disease.
- •
In patients with relapsed or refractory disease, the use of new drugs, with mechanisms of action based on peculiar biologic features of the tumor, will impact the ability to obtain disease control and enhance survival.
Introduction
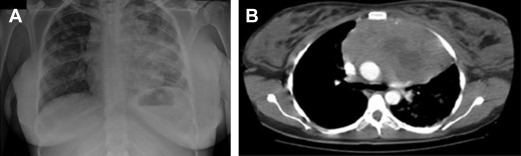
Primary mediastinal B-cell lymphoma (PMBCL) is a rather infrequent aggressive lymphoma, putatively arising from a transformed thymic B cell. It accounts for less than 5% of all non-Hodgkin lymphomas, and typically affects adolescents and young women in their third or fourth decade. It clinically presents with bulky mediastinal masses, usually exerting compressive effects on nearby vessels and airways ( Fig. 1 ), giving rise to the possible abrupt onset of dyspnea; dysphagia; thoracic pain; and facial, neck, breast, and arm edema ( Fig. 2 ), or infiltrating the adjacent lung parenchyma. It should be often regarded as a hematological emergency and promptly treated; the initial treatment decision is crucial for the management of this disease.

PMBCL was originally recognized as a subtype of diffuse large B-cell lymphoma (DLBCL) since the 1994 Revised European American Lymphoma (REAL) classification, and it has been regarded as a unique clinical and biological entity since the 2001 World Health Organization classification. Apart from its peculiar clinical presentation and pathologic features, this disease also displays a unique molecular fingerprint, which clearly distinguishes it from the rest of DLBCL. That same fingerprint, however, partly overlaps with the molecular profile of nodular sclerosis Hodgkin disease, with which it shares at least a third of its genes, abnormalities on chromosome 9p (in nearly three-quarters of cases), and CD30 expression (although weaker).
In the last 20 years, several studies—mostly retrospective in their fashion due to the rarity of the disease and to the difficulty of designing randomized clinical trials specifically addressed to these patients—have tried to provide answers to several issues regarding the optimal treatment and management of patients. Above all, although the necessity of an anthracycline-based induction is understood, the choice of the initial chemotherapy approach is still debated, including the value of the addition of rituximab. Although most of institutions have agreed to adopt an R-CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) induction (as they would do for any other subtypes of DLBCL) others prefer a more intense approach, either based on weekly administered third-generation regimens or on more aggressive strategies.
Apart from the debate on which first-line approach seems more suitable, there are other aspects that require clarification:
The actual role of external beam radiotherapy (RT), recognized as an adjuvant strategy consolidates the a response to chemotherapy, and the possibility of its omission in certain categories of patients to reduce the likelihood of radiation-induced long-term toxicity
The value of fluorodeoxyglucose (FDG) positron emission tomography (PET) scan and the way in which its results are interpreted in this peculiar context, either when performed at disease staging or after induction treatment
If PET itself can be regarded as a possible guide to the administration of a subsequent consolidation treatment, whether based on external RT or on a high-dose approach and autotransplant
The treatment of refractory and relapsing patients, for whom standard approaches have shown rather unsatisfactory results
Some new drugs are now emerging as potentially active agents in the setting of recurrent disease, as their mechanisms of action are based on specific biologic features of PMBCL.
Purpose of this article is to review the currently adopted strategies in the first-line treatment of PMBCL, to analyze the risks and benefits of postinduction RT consolidation, and to discuss the potential role of PET in helping physicians optimize the approach to their patients. A preview to some new agents on the horizon is also provided. A patient evaluation overview is provided in Box 1 .
- •
Histologically confirmed diagnosis of PMBCL made by an expert hematopathologist (excisional biopsy of a suspect mediastinal mass in a compatible clinical context)
- •
Full patient history, collection of concomitant medications, and complete physical examination (seek for superficial lymph nodes enlargement, jugular vein distension, thoracic edema, collateral veins, pleural effusion; inspect the oral cavity; listen to heart sounds and murmurs)
- •
Laboratory: full blood counts, creatinine, lactate dehydrogenase (LDH), transaminases, blood proteins with electrophoresis; serology for hepatitis B and C viruses and human immunodeficiency virus
- •
CT scan of neck, thorax, abdomen and pelvis (with contrast) and fluorodeoxyglucose PET scan
- •
Bone marrow biopsy (can be omitted in the event that an urgent treatment is required because of severe dyspnea, dysphagia, or superior vena cava syndrome)
- •
Transthoracic echocardiogram, to evaluate the fitness of the patient in receiving anthracyclines and to rule out the presence of pericardial effusion or possible cardiac tamponade
- •
PET scan is to be repeated 6 to 8 weeks after the end of the induction treatment, along with a full-body CT scan; interim PET scan evaluation is encouraged but not mandatory, as it is still considered investigational
Introduction
Primary mediastinal B-cell lymphoma (PMBCL) is a rather infrequent aggressive lymphoma, putatively arising from a transformed thymic B cell. It accounts for less than 5% of all non-Hodgkin lymphomas, and typically affects adolescents and young women in their third or fourth decade. It clinically presents with bulky mediastinal masses, usually exerting compressive effects on nearby vessels and airways ( Fig. 1 ), giving rise to the possible abrupt onset of dyspnea; dysphagia; thoracic pain; and facial, neck, breast, and arm edema ( Fig. 2 ), or infiltrating the adjacent lung parenchyma. It should be often regarded as a hematological emergency and promptly treated; the initial treatment decision is crucial for the management of this disease.

PMBCL was originally recognized as a subtype of diffuse large B-cell lymphoma (DLBCL) since the 1994 Revised European American Lymphoma (REAL) classification, and it has been regarded as a unique clinical and biological entity since the 2001 World Health Organization classification. Apart from its peculiar clinical presentation and pathologic features, this disease also displays a unique molecular fingerprint, which clearly distinguishes it from the rest of DLBCL. That same fingerprint, however, partly overlaps with the molecular profile of nodular sclerosis Hodgkin disease, with which it shares at least a third of its genes, abnormalities on chromosome 9p (in nearly three-quarters of cases), and CD30 expression (although weaker).
In the last 20 years, several studies—mostly retrospective in their fashion due to the rarity of the disease and to the difficulty of designing randomized clinical trials specifically addressed to these patients—have tried to provide answers to several issues regarding the optimal treatment and management of patients. Above all, although the necessity of an anthracycline-based induction is understood, the choice of the initial chemotherapy approach is still debated, including the value of the addition of rituximab. Although most of institutions have agreed to adopt an R-CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) induction (as they would do for any other subtypes of DLBCL) others prefer a more intense approach, either based on weekly administered third-generation regimens or on more aggressive strategies.
Apart from the debate on which first-line approach seems more suitable, there are other aspects that require clarification:
The actual role of external beam radiotherapy (RT), recognized as an adjuvant strategy consolidates the a response to chemotherapy, and the possibility of its omission in certain categories of patients to reduce the likelihood of radiation-induced long-term toxicity
The value of fluorodeoxyglucose (FDG) positron emission tomography (PET) scan and the way in which its results are interpreted in this peculiar context, either when performed at disease staging or after induction treatment
If PET itself can be regarded as a possible guide to the administration of a subsequent consolidation treatment, whether based on external RT or on a high-dose approach and autotransplant
The treatment of refractory and relapsing patients, for whom standard approaches have shown rather unsatisfactory results
Some new drugs are now emerging as potentially active agents in the setting of recurrent disease, as their mechanisms of action are based on specific biologic features of PMBCL.
Purpose of this article is to review the currently adopted strategies in the first-line treatment of PMBCL, to analyze the risks and benefits of postinduction RT consolidation, and to discuss the potential role of PET in helping physicians optimize the approach to their patients. A preview to some new agents on the horizon is also provided. A patient evaluation overview is provided in Box 1 .
- •
Histologically confirmed diagnosis of PMBCL made by an expert hematopathologist (excisional biopsy of a suspect mediastinal mass in a compatible clinical context)
- •
Full patient history, collection of concomitant medications, and complete physical examination (seek for superficial lymph nodes enlargement, jugular vein distension, thoracic edema, collateral veins, pleural effusion; inspect the oral cavity; listen to heart sounds and murmurs)
- •
Laboratory: full blood counts, creatinine, lactate dehydrogenase (LDH), transaminases, blood proteins with electrophoresis; serology for hepatitis B and C viruses and human immunodeficiency virus
- •
CT scan of neck, thorax, abdomen and pelvis (with contrast) and fluorodeoxyglucose PET scan
- •
Bone marrow biopsy (can be omitted in the event that an urgent treatment is required because of severe dyspnea, dysphagia, or superior vena cava syndrome)
- •
Transthoracic echocardiogram, to evaluate the fitness of the patient in receiving anthracyclines and to rule out the presence of pericardial effusion or possible cardiac tamponade
- •
PET scan is to be repeated 6 to 8 weeks after the end of the induction treatment, along with a full-body CT scan; interim PET scan evaluation is encouraged but not mandatory, as it is still considered investigational
First-line chemotherapy combinations in prerituximab era: different perspectives
Since prerituximab era, the first-line chemotherapy schedule has represented a matter of controversy. Although the CHOP regimen was mainly adopted by US centers, the European experience has carried out evidence that MACOP-B (methotrexate, doxorubicin, cyclophosphamide, vincristine, bleomycin, prednisone) or VACOP-B (etoposide, doxorubicin, cyclophosphamide, vincristine, bleomycin, prednisone), both weekly dosed third-generation dose-dense regimens, could be superior to CHOP. As a consequence of the application of dose-dense regimens, in fact, remission rates and survival functions have appeared to be at least as good as—or probably even better than—those observed for DLBCL patients, thus retracting the initial impression that PMBCL was per se a prognostically unfavorable subset of DLBCL.
However, this conclusion was only drawn from existing reports, since no randomized clinical trial has been carried on so far, and prospective studies are also lacking ( Table 1 ). Overall it is clear that an anthracycline-containing regimen should be regarded as the first approach to PMBCL.
| Author, Year | Treatment | Patients | CR, % | PFS or RFS, % |
|---|---|---|---|---|
| Zinzani et al, 1996 | (F)-MAC(H)OP-B + RT | 22 | 95 | 89 (62 mo) |
| Lazzarino et al, 1997 | — | 106 | — | — |
| CHOP ± RT | — | 36 | 38 (36 mo) | |
| M(V)ACOP-B ± RT | — | 73 | 58 (36 mo) | |
| Zinzani et al, 1999 | MACOP-B + RT | 50 | 86 | 93 (96 mo) |
| Zinzani et al, 2001 | MACOP-B + RT | 89 | 88 | 91 (108 mo) |
| Zinzani et al, 2002 | — | 426 | — | — |
| CHOP ± RT | 105 | 61 | 35 (120 mo) | |
| Third-generation a ± RT | 277 | 79 | 67 (120 mo) | |
| High-dose/autoSCT ± RT | 44 | 75 | 78 (120 mo) | |
| Todeschini et al, 2004 | — | 138 | — | — |
| CHOP ± RT | 43 | 51 | 40 (120 mo) | |
| M(V)ACOP-B ± RT | 95 | 80 | 76 (120 mo) | |
| Savage et al, 2006 | CHOP, CHOP-like ± RT | 63 | n.r. | 71 (60 mo) |
| M(V)ACOP-B ± RT | 47 | n.r. | 87 (60 mo) b | |
| Mazzarotto et al, 2007 | Third-generation a ± RT | 53 | 89 | 85 (94 mo) |
| De Sanctis et al, 2008 | MACOP-B + RT | 92 | 87 | 81 (160 mo) |
a Third-generation treatments include: MACOP-B, VACOP-B and ProMACE-CytaBOM.
Lazzarino and colleagues first showed the MACOP-B/VACOP-B superiority on CHOP both in terms of complete response (CR) rates and relapse-free survival (RFS), with a 73% CR rate for the former treatment versus 36% for the latter treatment, and a 3-year RFS of 58% versus 38%. The same issue was confirmed by a subsequent retrospective multicenter Italian experience of 138 patients. Patients on MACOP-B/VACOP-B achieved better results than those on CHOP, both with CR and event-free survival (EFS) rates, with a statistically significant difference in low and low–intermediate International Prognostic Index (IPI) risk groups (into which most patients with PMBCL generally fall), although lacking significance in high–intermediate-risk and high-risk disease.
The results of a multinational retrospective analysis published in 2002 by the International Extranodal Lymphoma Study Group (IELSG) clearly showed that patients treated with third-generation regimens or high-dose therapy performed better than those who received a first-generation approach (ie, CHOP, Fig. 3 ), and that RT (delivered to 80% of the enrolled patients) played a pivotal role in the consolidation of response, as it was able to convert partial responses into CR, as documented with a gallium ( 67 Ga) scan.
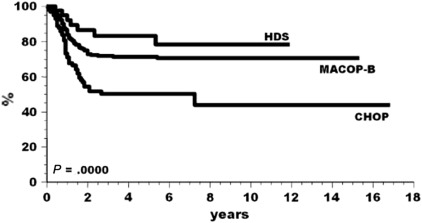
On the basis of these results, MACOP-B or VACOP-B induction, followed by mediastinal RT, was thus suggested to be a suitable induction strategy for PMBCL patients. Nevertheless, the effective superiority of third-generation treatments over CHOP and CHOP-like regimens has never been validated, either in larger phase 3 studies or in randomized trials. Alternative strategies, such as the application of high-dose methotrexate, have yielded similar response rates, although with a significantly higher incidence of toxic events and hospitalization rates.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree