Diffuse large B-cell lymphoma presents as limited-stage disease in approximately 30% of cases. Historically, therapy relied on a combined modality of abbreviated chemotherapy followed by involved-field radiotherapy (IFRT). Due to the apparent lack of long-term survival and the concern for delayed toxicity, chemotherapy-only strategies are used more frequently. Treatment should take into account patient performance, clinical risks, and involvement sites. PET-guided approaches are being investigated. The risk of late relapse has been recognized, highlighting the importance of long-term follow-up. Future efforts must incorporate biological features to improve risk assessment, guide clinical decisions, and achieve an individualized therapy.
Key points
- •
Limited-stage diffuse large B-cell lymphoma (DLBCL) patients usually present with a favorable clinical risk profile. In the rituximab era, patients with 0 to 1 risk factors have a survival rate greater than 90% at 5 years.
- •
Therapeutic approaches include combined-modality (immunochemotherapy and radiotherapy) versus immunochemotherapy-alone strategies. A PET-guided approach may facilitate individualized therapy.
- •
Current evidence does not support the requirement for radiotherapy in all patients. Patients with poor chemotherapy tolerance or evidence of chemotherapy resistance may benefit from the inclusion of radiotherapy.
- •
Late relapses can occur in limited-stage DLBCL. Repeat biopsy at relapse is highly recommended to guide further management and to enable ongoing translational research.
Introduction
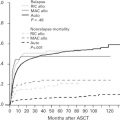
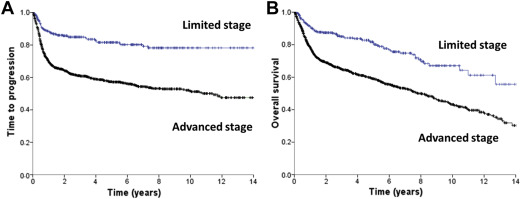
Diffuse large B-cell lymphoma (DLBCL) presents as localized disease in about 25% to 30% of patients, which is of clinical relevance both in terms of prognosis and treatment. Using a practical definition of limited stage, including patients who present with Ann Arbor stages I or II nonbulky (<10 cm) disease without B-symptoms, these cases have a superior outcome compared with advanced-stage patients ( Fig. 1 ). Limited-stage DLBCL patients typically present with no or few clinical risk factors, which contributes to the favorable outcome observed. For this reason, a stage-modified International Prognostic Index (IPI) was specifically proposed for these cases.
Currently, there is no standard management approach for limited-stage DLBCL. A variety of treatment options exist, relying on either short or full-length courses of immunochemotherapy, with or without radiotherapy. However, the role of radiotherapy in the rituximab era must be redefined and the use of PET-guided strategies should be explored. At present, overall outcome is excellent with expected 5-year overall survival (OS) and progression-free survival (PFS) above 90% and 80%, respectively. However, a delayed pattern of relapse has been observed and seems to persist despite the introduction of rituximab. Whether this reflects a unique biology for limited-stage DLBCL deserves further investigation. The aim of this article is to review the key literature in limited-stage DLBCL and how it may serve to guide current practice. Central nervous system, testicular, and primary mediastinal B-cell lymphoma are excluded because their unique biological and clinical features require specific consideration.
Introduction
Diffuse large B-cell lymphoma (DLBCL) presents as localized disease in about 25% to 30% of patients, which is of clinical relevance both in terms of prognosis and treatment. Using a practical definition of limited stage, including patients who present with Ann Arbor stages I or II nonbulky (<10 cm) disease without B-symptoms, these cases have a superior outcome compared with advanced-stage patients ( Fig. 1 ). Limited-stage DLBCL patients typically present with no or few clinical risk factors, which contributes to the favorable outcome observed. For this reason, a stage-modified International Prognostic Index (IPI) was specifically proposed for these cases.
Currently, there is no standard management approach for limited-stage DLBCL. A variety of treatment options exist, relying on either short or full-length courses of immunochemotherapy, with or without radiotherapy. However, the role of radiotherapy in the rituximab era must be redefined and the use of PET-guided strategies should be explored. At present, overall outcome is excellent with expected 5-year overall survival (OS) and progression-free survival (PFS) above 90% and 80%, respectively. However, a delayed pattern of relapse has been observed and seems to persist despite the introduction of rituximab. Whether this reflects a unique biology for limited-stage DLBCL deserves further investigation. The aim of this article is to review the key literature in limited-stage DLBCL and how it may serve to guide current practice. Central nervous system, testicular, and primary mediastinal B-cell lymphoma are excluded because their unique biological and clinical features require specific consideration.
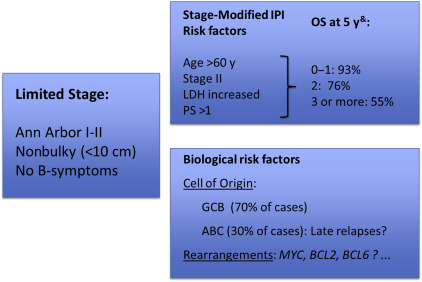
Definition of limited-stage diffuse large B-cell lymphoma and prognostic factors
Limited-stage DLBCL, similar to its pseudonyms, early-stage or localized DLBCL, lacks a precise definition. Limited stage is typically defined as patients who present with Ann Arbor stages I or II nonbulky disease that is confinable within a radiation field. Additional risk factors, such as lack of B-symptoms, have also been incorporated within the definition; however, these have varied among different studies, making cross-trial comparisons difficult. Similarly, the threshold used to define bulk has varied between the studies, with 10 cm most commonly used, which may further affect reported outcomes.
Because most limited-stage DLBCL patients present with a favorable risk profile, the IPI is of limited utility. As such, investigators of the Southwest Oncology Group (SWOG) introduced a stage-modified IPI to better stratify patients according to risk of progression and survival ( Table 1 ). The stage-modified index retains age, lactate dehydrogenase, and performance status as risk factors but redefines stage II as a negative prognostic factor and eliminates the number of extranodal sites, which is applicable only for advanced disease. Of note, the stage-modified IPI has been validated in retrospective series both in the pre-rituximab and rituximab era. More recently, the newly described National Comprehensive Cancer Network (NCCN)-IPI has also been evaluated in a retrospective series of localized DLBCL and may be the most powerful prognostic index in patients receiving immunochemotherapy.
| Risk Factor | Risk Group | Pre-Rituximab Era | Rituximab Era |
|---|---|---|---|
| 5-y OS (%) | 3-y OS (%) | ||
| IPI , a | |||
| Age >60 y LDH >Normal Stage III-IV PS 2–4 Extranodal >1 | Low (0–1) | 73 | 91 |
| Low-intermediate (2) | 51 | 81 | |
| High-intermediate (3) | 43 | 65 | |
| High (4–5) | 26 | 59 | |
| R-IPI , a | |||
| Very good (0) | — | 94 | |
| Good (1–2) | — | 79 | |
| Poor (3–5) | — | 55 | |
| aa-IPI , a | |||
| LDH >Normal Stage III-IV PS 2–4 | Low (0) | 83 | 98 |
| Low-intermediate (1) | 69 | 92 | |
| High-intermediate (2) | 46 | 75 | |
| High (3) | 32 | 75 | |
| Stage-Modified IPI | |||
| Age >60 y Stage II LDH Increased PS >1 | 0–1 risk factor | 82 | 93 b |
| 2 risk factors | 71 | 76 b | |
| 3 risk factors | 48 | 55 b | |
| NCCN-IPI , a | |||
| Low 0–1 L-I 2–3 H-I 4–5 High ≥6 | — — — — | 94 72 54 35 | |
a IPI, R-IPI, aa-IPI, and NCCN-IPI survival data include advanced-stage DLBCL. Survival data extracted from original prognostic index publications, Ref. 5, and unpublished results from British Columbia Cancer Agency (BCCA) series of limited-stage DLBCL treated in the rituximab era.
b 5-year OS in the BCCA series of limited-stage DLBCL subjects (n = 361).
c Extranodal disease in bone marrow, central nervous system, liver or gastrointestinal tract, or lung.
Based on the stage-modified IPI, patients with 0 or 1 adverse clinical risk factors have an excellent outcome, with an OS of more than 90% at 5 years. Consideration of less intensive strategies may be appropriate for this subgroup. In contrast, patients with 2 or more clinical risk factors have a moderately favorable outcome, with a 5-year OS of approximately 75%. Identifying the 20% to 25% of patients who will ultimately fail is an important challenge because this would allow for an individualized treatment approach. Improved insight into the biology of DLBCL and the availability of molecular markers to enable better prognostication will be paramount for this group ( Fig. 2 ).
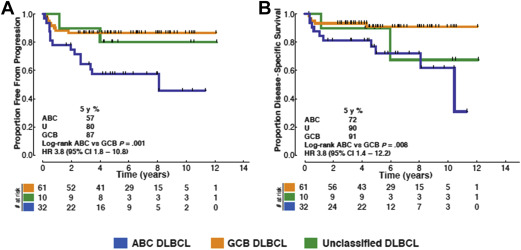
The observation that limited-stage DLBCL patients more frequently exhibit delayed relapses compared with advanced-stage patients has led to the speculation that this may represent unique biology. To date, there are few data evaluating the biological differences between limited-stage and advanced-stage patients. In a recent retrospective analysis using the Lymph2Cx assay to determine cell of origin (COO), patients with limited-stage DLBCL were found to have predominantly a germinal center B-cell (GCB) subtype, representing approximately 70% of cases. In addition, similar to what has been shown largely for advanced-stage cases, the activated B-cell (ABC) subtype is associated with worse outcomes. Interestingly, it seems that delayed relapses are not observed in the GCB subgroup ( Fig. 3 ). A recent study using the Hans algorithm for COO classification has also observed a higher proportion of GCB subtype (75%) in limited-stage DLBCL, although no difference in outcome according to COO was observed, possibly due to the limitations of classification by immunohistochemistry. At present, there is no information on the frequency or prognostic impact of MYC , BCL2 , or BCL6 rearrangements within limited-stage DLBCL patients and it is unknown whether this should influence treatment decisions.
Clinical studies in the pre-rituximab era
Most of the therapeutic evidence collected from randomized clinical trials in limited-stage DLBCL comes from studies conducted before the introduction of rituximab ( Table 2 ). Undoubtedly, among the 4 randomized trials comparing combined modality approaches with radiotherapy to chemotherapy alone, the most influential was the SWOG 8736 (S8736) study, which demonstrated the superiority at 5 years of 3 cycles of cyclophosphamide, doxorubicin, vincristine, and prednisone CHOP followed by IFRT compared with 8 cycles of CHOP alone. The initial results of this study not only confirmed the lesser toxicity of a combined modality approach over full-length CHOP but also its superiority in terms of local control of disease and OS. This resulted in the broad adoption of 3 cycles of CHOP and IFRT as the standard of care for limited-stage DLBCL. However, in an updated analysis, the improvement in PFS and OS with abbreviated chemotherapy and IFRT disappeared at 7 to 9 years of follow-up due to a higher number of delayed relapses and death from lymphoma in that cohort. In the final analysis of S8736 with a median follow-up time of 17.7 years, the 2 arms had almost an identical median OS of approximately 13 years and a similar pattern of delayed relapses without a plateau being observed. Although this trial suggests that the use of IFRT may offer a short-term advantage, it also raises the concern that abbreviated chemotherapy may be insufficient for some patients because most relapses in the combined modality arm occurred outside of the radiation field.
| Author | Study | Subjects (N) | Treatment | 5-y PFS | 5-y OS | Benefit for RT P (<.05) |
|---|---|---|---|---|---|---|
| Studies in the Pre-Rituximab Era | ||||||
| Miller et al, 1998 | SWOG 8736 | 395 a | CHOP × 3 + IFRT | 77 | 82 | Yes (PFS & OS) |
| CHOP × 8 | 64 | 72 | ||||
| Stephens et al, 2016 | SWOG 8736 (final subset analysis) | 308 b | CHOP × 3 + IFRT | 11.1 y h | 13.7 y h | No |
| CHOP × 8 | 12 y h | 13 y h | ||||
| Horning et al, 2004 | ECOG 1484 | 172 c (in CR) | CHOP × 8 + IFRT | 73 i | 87 | Disease-free survival only |
| CHOP × 8 | 56 | 73 | ||||
| Reyes et al, 2005 | GELA LNH-93–1 | 647 d | CHOP × 3+ IFRT | 74 j | 81 | No k |
| ACVBP | 82 | 90 | ||||
| Bonnet et al, 2007 | GELA LNH-93–4 | 576 e | CHOP × 4 + IFRT | 64 j | 68 | No |
| CHOP × 4 | 61 | 72 | ||||
| Studies in the Rituximab Era | ||||||
| Persky et al, 2008 | SWOG 0014 | 60 f | R4 + CHOP × 3 + IFRT | 88% at 4 y | 92% at 4 y | — |
| Persky et al, 2015 | SWOG S0313 | 46 f | CHOP × 3 + IFRT + ibritumomab | 82% at 5 y | 87% at 5 y | — |
| Lamy et al, 2014 | Lysa/Goelams | 313 g | R-CHOP × 4 or 6 + IFRT | 91 j | 95 | No |
| Phase III 02–03 | R-CHOP × 4 or 6 | 87 | 90 | |||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree