Oncologic Emergencies
Jason L. Freedman
Susan R. Rheingold
Michael J. Fisher
Emergencies can occur at any time during a child’s course of care for cancer. Some emergencies are the initial manifestation of cancer or develop as the diagnosis is being made; others arise as a consequence of therapy, and some develop at the time of cancer progression or recurrence. All physicians who care for children with cancer must be able to recognize life-threatening emergencies and triage or treat them quickly and appropriately.
This chapter addresses pediatric oncologic emergencies by system—cardiothoracic, gastrointestinal (GI), genitourinary, neurologic, vascular, metabolic, and endocrine; as well, it addresses therapy-associated emergencies and summarizes the etiology and differential of cardiovascular collapse and shock. Chapter 39 reviews the management of emergencies associated with cytopenias and abnormal hemostasis and use of blood component therapy; Chapter 40 covers infectious complications; and Chapter 42 covers the principles of pain management.
CARDIOTHORACIC EMERGENCIES
Respiratory distress is a common presenting symptom of intrathoracic malignancies and is often the sole symptom of a cardiothoracic emergency occurring at any time. Respiratory distress can be caused by mediastinal lesions compressing the airway and vasculature (superior vena cava syndrome [SVCS] and superior mediastinal syndrome [SMS]); intrapulmonic processes such as infiltrates, pneumothoraces, masses, and fibrosis; intrapleural processes such as masses and effusions; and cardiac processes such as masses, effusions, fibrosis, and failure.
SVCS and SMS
SVCS refers to the signs and symptoms resulting from compression, obstruction, or thrombosis of the superior vena cava. The term SMS is used when tracheal compression also occurs. In children with mediastinal masses, tracheal compression and respiratory embarrassment usually coexist with SVCS; therefore, SVCS and SMS are often used synonymously and herein are referred to as SVCS.
Etiology and Pathogenesis
The most common primary cause of SVCS in children is compression by malignant mediastinal masses, with thrombotic complications of implantable intravascular devices now the second most common etiology.1,2 Mediastinal granulomas from tuberculosis and infections such as histoplasmosis are rare causes of SVCS in developed countries, but are symptomatically indistinguishable from other etiologies. In the pediatric population, the most frequent malignant etiologies of mediastinal masses are non-Hodgkin lymphoma (NHL), Hodgkin lymphoma (HL) and T-cell acute lymphoblastic leukemia (ALL).2,3,4 In a retrospective review, SVCS occurred most often with those mediastinal masses associated with germ cell tumors, followed by sarcomas, ALL, NHL, and Hodgkin lymphoma.3
The SVC is a thin-walled vessel with low intraluminal pressure that carries approximately one-third of venous return to the heart. Tumor or infection in the mediastinal or paratracheal lymph nodes or thymus can compress the SVC, causing venous stasis. The adjacent pericardium and coronary or collateral vessels can also fill with tumor or clot. Compression, thrombosis, and edema combine to minimize tracheobronchial airflow and reduce venous return from the head, neck, and upper thorax, causing the signs and symptoms of both SVCS. The trachea and right main stem bronchus in young children are more compressible than in adults, making the symptoms of compression especially pronounced in infants and toddlers.2
Evaluation
The most common symptoms of SVCS in children are dyspnea, cough, wheeze, and orthopnea (Table 38.1). Symptoms are typically aggravated when the patient is supine, such as for a computed tomographic (CT) scan, bone marrow aspiration, or lumbar puncture (LP).1,2,3,5,6,7 Anxiety, confusion, lethargy, headache, distorted vision, and syncope are rare but indicate carbon dioxide retention and central venous stasis and require immediate intervention. Characteristic physical findings include head and neck edema, plethora, and cyanosis of the face, neck, and upper extremities; cervical and thoracic venous distention; conjunctival suffusion and edema; and wheezing or stridor.1-3, 5-7 In children and adolescents, the symptoms often progress rapidly over days.
TABLE 38.1 Symptoms and Physical Findings in Patients with Superior Vena Cava Syndrome at Initial Presentation | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||
Management of children with mediastinal malignancies can be challenging, as respiratory distress and cardiovascular compromise are often the presenting symptoms. A child with a single or many of these signs and symptoms needs both a posterior-anterior and lateral chest radiography (CXR). Most children with SVCS will have a mass in the anterior superior mediastinum. Pleural and pericardial effusions are more common in NHL than in HL or other malignancies.7 A CXR may show tracheal deviation and main stem bronchial compression. CT scan with IV contrast can delineate the distortions of normal anatomy, assess the vasculature, and more accurately assess the extent of tracheal compression.2,8,9 Figure 38.1 shows compression of the trachea and superior vena cava by an anterior mediastinal lymphoma. The CT scan can be obtained in the prone position if the supine position aggravates the respiratory distress. If there is concern that the symptoms are being caused by a thromboembolism or pericardial effusion, an echocardiogram should be obtained. Anesthesiologists often require an echocardiogram before sedating a patient with a mediastinal mass to assess cardiac or great vessel compression from mass and presence of pericardial effusion.6,10 Pulmonary function tests and volume flow loop assess pulmonary reserve and resilience, but as they do not seem to correlate with outcome, they are performed less frequently.
When cancer is the probable cause of SVCS, it is desirable to obtain a tissue specimen for diagnosis. It is imperative that the diagnosis is made by the least invasive procedure possible, as respiratory and cardiovascular failure may occur with sedation or general anesthesia.5,6,7,8,10 During general anesthesia, respiratory muscle tone decreases, abdominal muscle tone increases, the caudal movement of the diaphragm disappears, bronchial smooth muscle relaxes, and lung volume diminishes.5 These changes aggravate the effects of extrinsic compression of the vena cava. Tracheal intubation may be extremely difficult, even impossible, and some patients will not tolerate extubation until the tumor bulk has been reduced. Conscious sedation or anxiolytics may also be contraindicated as they decrease respiratory drive and dilate peripheral vessels, thereby reducing venous return.
Standardized criteria to predict the severity and complications of SVCS do not exist. Studies have evaluated anesthetic complication risk according to tracheal cross section on CT, mediastinal mass width on CT, and pulmonary function testing.6 Patients with tumors greater than 45% of the transthoracic diameter are more likely to be symptomatic than patients having smaller tumors with ratios less than 30%.7 Tracheal cross-sectional area less than 50% of predicted and peak expiratory flow rate less than 50% of predicted value are associated with poor anesthetic risk.4,6,7,8 Great vessel and tracheal compression noted on CT and increasing number of respiratory signs and symptoms have also been predictive of anesthetic complications.5,10 Although no single measure is predictive of anesthetic outcome, they can all provide important information in regard to risk.
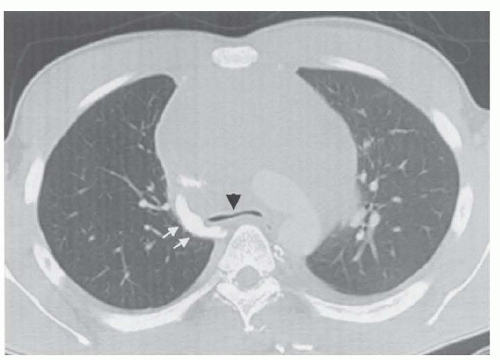 Figure 38.1 CT scan of the chest revealing compression of the trachea (black arrow head) and superior vena cava (white arrows) by a mediastinal lymphoma. |
Diagnosis should be made in the least invasive manner possible, and a stepwise approach is recommended. An algorithm appropriate for pediatric patients is shown in Figure 38.2. A complete blood cell count (CBC) may reveal blasts, and flow cytometry from the peripheral blood can be diagnostic. A bone marrow aspiration performed with local anesthesia may reveal leukemic blasts. Pleurocentesis or pericardiocentesis may offer immediate relief and provide diagnostic material by cytology or cytogenetics. If there is an enlarged peripheral lymph node, node biopsy is faster and less invasive than a mediastinal biopsy. Anterior mediastinal biopsies can be performed under local anesthesia via anterior mediastinoscopy or mediastinotomy using ultrasound (US) or CT guidance in older children.2,11,12
Therapy
In a child who presents with SVCS as an initial symptom of malignancy, establishing a tissue diagnosis may be impossible; it may be medically necessary to start empiric therapy. Historically, emergency therapy was irradiation, which often resulted in rapid improvement in symptomatology because leukemias and lymphomas are exquisitely radiosensitive. Due to rapid response rates with decreased toxicity, empiric systemic steroids and chemotherapy for suspected leukemic and lymphomatous masses are now the standard of care.2,13 Intravenous steroids (methylprednisolone or dexamethasone) should be started immediately in a child in distress if a diagnostic procedure cannot be performed or if a preliminary diagnosis is known by noninvasive means. The majority of tumor types causing SVCS are sensitive to cyclophosphamide (CPM), vincristine, and/or an anthracycline, which are readily available at all oncology centers. Tumor lysis precautions should be initiated to prevent the metabolic and renal complications of rapid tumor dissolution (see subsequent discussion on tumor lysis syndrome).
For tumors not responsive to chemotherapy, radiation is often indicated. Radiation oncologists use highly focused radiation portals, such as a small field centered on the trachea only to circumvent the problem of swelling of small airways. Another option consists of using small bilateral opposing fields encompassing the trachea, superior vena cava, and proximal right auricle. The daily dose is governed by the presumed radiosensitivity of the tumor. The phenomenon of postirradiation deterioration seems limited to children and adolescents, perhaps because of the greater compressibility of their respiratory structures and the inability of their more narrow lumina to accommodate postirradiation edema. If the patient’s symptoms worsen because of airway edema during empiric irradiation, IV methylprednisolone (1 mg/kg) every 6 hours may be unavoidable. Less responsive neoplasms such as teratoma, neuroblastoma, germ cell tumor, or benign tumors may not respond quickly to radiation therapy, making surgical resection inevitable.
Unfortunately, empiric chemotherapy or radiation can confound the diagnosis, as it may render the histologic picture uninterpretable within 48 hours. Loeffler and associates reported that emergency prebiopsy irradiation rendered the histologic specimen uninterpretable in 8 of 19 patients with a mediastinal mass; however, the authors point out that patient management was not altered by prebiopsy therapy.14 Borenstein et al.13 reviewed the diagnostic outcome of 23 children with mediastinal lymphoma treated with IV steroids prior to biopsy due to respiratory compromise. Of the 23 children, steroids had an adverse effect on diagnosis in 5 (22%). Two had a delay in diagnosis, two had no definitive diagnosis and were treated presumptively, and one had a failure of
staging.13 Failure to persist with treatment for a presumed leukemia or lymphoma, even if a histologic diagnosis is not made, may allow the disease to progress to a more advanced stage.14
staging.13 Failure to persist with treatment for a presumed leukemia or lymphoma, even if a histologic diagnosis is not made, may allow the disease to progress to a more advanced stage.14
In emergency situations or with vascular compromise, arterial extracorporeal membrane oxygenation has been used successfully to support a patient through diagnosis and early treatment with chemotherapy.15 Tracheal stents have also been placed in emergency situations or for end-stage palliation.2,16
If signs or symptoms of SVCS develop in a child with a CVL and no mediastinal mass, one must evaluate for a venous thromboembolism (VTE). Although often asymptomatic, CVL-associated VTEs should be treated with anticoagulation in children with cancer.17,18 In children who are symptomatic, infusional thrombolytic therapy may be indicated. Fibrinogen, fibrin split products, platelet count, prothrombin time (PT), and activated partial thromboplastin time (aPTT) should be monitored closely during thrombolysis. Systemic or low-molecular-weight heparin (LMWH) therapy begins either during or immediately following thrombolytic therapy. LMWH is rapidly becoming the antithrombotic of choice in children with cancer due to its better safety profile and lack of drug interaction. LMWH should be started at 1 mg/kg BID in children older than 2 years, with higher doses for younger children.18 Goal antifactor Xa levels are 0.5 to 1 U/mL.18 Placement of a CVL into the SVC should be delayed until SVCS resolves.
Pleural and Pericardial Effusions
Etiology and Pathogenesis
An effusion represents the escape of fluid into a potential space and can be exudative or transudative. Exudates are generally caused by malignancy or infection and result from inflammation.19 In contrast, transudates result from a sympathetic response to tumor in the chest or abdomen, fluid overload, heart failure, or hypoproteinemia. Exudates have protein concentrations greater than 2.5 g/dL, a specific gravity greater than 1.015, and a high cell count. Protein concentration, specific gravity, and cell counts are low in transudates.
Evaluation
Symptoms of both pericardial and pleural effusions include dyspnea, tachypnea, orthopnea, chest pain, retractions, and cough.19,20,21 Small, clinically silent pleural and pericardial effusions are often detected incidentally on radiographs and echocardiograms. Although an asymptomatic effusion may not require intervention, a large accumulation of pericardial fluid can cause cardiac tamponade and rapid decompensation. Emergency thoracentesis or pericardiocentesis can relieve respiratory or cardiac distress, provide diagnostic fluid, and eliminate a potential reservoir for drugs such as methotrexate. Laboratory evaluation of the fluid should include cell count, protein content, cytology, Gram staining, culture, cytogenetics, and assays of appropriate immunologic and biologic markers.
Therapy
In a child with untreated cancer and respiratory distress, removal of the fluid one time often suffices, as the fluid usually does not reaccumulate once therapy is under way. In contrast, children with advanced malignant disease may develop recurrent effusions that compromise their duration and quality of life. Palliative measures include repeated centesis, placement of an indwelling percutaneous catheter, or thorascopic instillation of a sclerosing agent into pleural or pericardial cavities to cause irritation and adhesion of
the potential space.19,22 Historically, talc and tetracycline were commonly used sclerosing agents for both pleural and pericardial effusions but are now difficult to obtain. Bleomycin and cisplatin have both been used successfully and safely in the treatment of malignant pleural effusions and their underlying malignancy.23 Instillation of cisplatin at 100 to 200 mg/m2 in warm saline with thiosulfate rescue into the thoracic or abdominal cavities has eliminated the intracavitary disease and secondary effusions/ascites.23 If sclerosing agents fail, pleural abrasion, surgical pleurectomy, or pericardiectomy may be necessary, but surgery risks substantial morbidity and potential mortality for patients with resistant metastatic disease.22
the potential space.19,22 Historically, talc and tetracycline were commonly used sclerosing agents for both pleural and pericardial effusions but are now difficult to obtain. Bleomycin and cisplatin have both been used successfully and safely in the treatment of malignant pleural effusions and their underlying malignancy.23 Instillation of cisplatin at 100 to 200 mg/m2 in warm saline with thiosulfate rescue into the thoracic or abdominal cavities has eliminated the intracavitary disease and secondary effusions/ascites.23 If sclerosing agents fail, pleural abrasion, surgical pleurectomy, or pericardiectomy may be necessary, but surgery risks substantial morbidity and potential mortality for patients with resistant metastatic disease.22
Cardiac Tamponade
Etiology and Pathogenesis
Cardiac tamponade occurs when the left ventricle fails to maintain output because of compression by pericardial fluid or leukemic infiltration, inflammation or infection of the pericardium, constrictive fibrosis from previous radiation, or occlusion from tumors of the cardiac muscle or endocardium.20 Kuhn et al.21 report that approximately one-third of pediatric patients with pericardial effusions have an underlying malignancy. The effusion may be due to the tumor itself or caused by infection in an immunocompromised host. A Wilms tumor thrombus can extend from the renal vein through the tricuspid valve to fill the right cardiac chambers. Although malignancy may cause pericardial effusions at diagnosis, cardiac tamponade is rarely the presenting symptom of an undiagnosed malignancy.20
Evaluation
Gradual accumulation of fluid allows the pericardium to accommodate a large volume, but rapid accumulation of several hundred milliliters can cause sudden decompensation. Symptoms of impending tamponade resemble those of congestive heart failure (CHF): cough, chest pain, dyspnea, hiccups, and abdominal pain. Signs include tachycardia, cyanosis, hypotension, and a pulsus paradoxus of more than 10 mm. Tamponade must be differentiated from CHF, infectious pericarditis or myocarditis, and therapy-induced cardiomyopathy. Constrictive pericarditis may cause friction rubs, diastolic murmurs, and atrial arrhythmias.
Radiographs of large pericardial effusions often show a typical “water bottle” cardiac shadow on anterior/posterior view and an abnormal space between the pericardial fat and pericardium on the lateral view. A mediastinal mass and pleural effusions may also be noted. CT scan often demonstrates compression of the cardiac chambers by the effusion, but can also reveal nodular lesions, pericardial thickening, and hemorrhagic effusion.1,9 The electrocardiogram (ECG) may show low-voltage QRS complexes, flattened or inverted T waves, and electrical alternans due to swinging heart. Echocardiography is the preferred diagnostic technique, as it can show the size of the pericardial effusion as well as right atrial and/or ventricular collapse. An echocardiography may also reveal a thickening of the pericardium consistent with pericarditis or pericardial tumor.1
Therapy
Supportive care for malignant pericardial effusion and constrictive pericarditis consists of hydration, oxygen, and positioning the patient to maximize cardiac output. Diuretics are contraindicated as hypovolemia decreases stroke volume. Definitive treatment of tamponade caused by an effusion is immediate removal of fluid by percutaneous pericardiocentesis with or without drain placement.1,9,21 Pericardiotomy or pericardial window and intrapericardial infusion of steroids, cisplatin, or bleomycin have been reported as therapeutic alternatives for patients with persistent symptomatic effusion unresponsive to medical management and tamponade from constrictive pericarditis.21,24 Pericardial fluid should be sent for protein, cell count, gram stain, culture, cytogenetics, and cytology. In patients with a Wilms tumor thrombus extending into the right side of the heart, if the mass does not occlude the chambers, therapy with dactinomycin and vincristine may reduce the size of thrombus within a week (see Chapter 29).
Massive Hemoptysis
Etiology and Evaluation
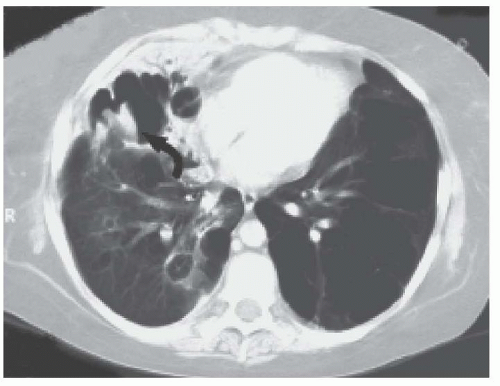
Massive blood loss into the respiratory tree can cause thrombus formation within the bronchial tree, leading to asphyxiation or, less likely, exsanguination. In general, pediatric tumors do not cause massive hemoptysis. The most common etiology of mild hemoptysis in an oncology patient is aspiration of blood from epistaxis. The most common cause of massive hemoptysis is invasive pulmonary aspergillosis (IPA). The underlying lesion may consist of a mycetoma cavity surrounded by many collateral vessels from the bronchial, axillary, or subclavian arteries. The majority of patients with IPA leading to massive hemoptysis are patients with acute leukemia recovering from a period of prolonged neutropenia.25,26 The increasing white blood cell (WBC) count accelerates the process of necrosis and increases the risk of hemorrhage by arterial perforation. A CXR often reveals a nodular or cavitary lesion or a peripheral wedge-shaped infiltrate. Thoracic CT may reveal a halo sign, a localized infiltrate with a halo of ground glass appearance, and an air-crescent sign, representing late cavitation.25,26 Figure 38.3 shows pulmonary aspergillosis in a patient with hemoptysis. The differential diagnosis includes all invasive fungi and, less frequently, bacterial pneumonias and consolidations with invasive organisms such as Staphylococcus aureus, Klebsiella, and Pseudomonas. A CBC, along with a PT, aPTT, fibrinogen, and fibrin split products, should be obtained immediately to look for coagulation abnormalities.
Therapy
Therapeutic objectives are to prevent asphyxiation, localize the site of the bleeding, and arrest hemorrhage. Thrombocytopenia and coagulopathy should be corrected and erythrocytes transfused as needed. For epistaxis, start with direct pressure by external compression of the nares with the patient in an upright position. Unreconstituted topical thrombin powder can be rubbed directly into the bleeding nare for local hemostasis. It should not be given intravenously. For repetitive or persistent epistaxis, an otolaryngologist should be consulted for proper packing or cautery.
If the site of pulmonary bleeding is known, the patient should lie on the side of the hemorrhage to prevent collection of blood into the unaffected lung. Preventing asphyxiation may require intubation. Systemic antifungal therapy that includes aspergillosis and mucor coverage should be initiated. When the patient has been stabilized, a chest CT should be obtained to localize the lesion. Bronchial artery embolization using selective intrabronchial coagulation with embolic agents such as polyvinyl alcohol particles or spherical embolic agents, or occlusion of the hemorrhaging vessel with a balloon catheter have met with success in patients with massive pulmonary hemorrhage from causes other than Aspergillus.26,27 Unfortunately, these procedures are rarely successful in abating hemoptysis from Aspergillus with its extensive collateral vessel network. Caillot and colleagues report very good outcomes with prophylactic resection of large aspergillomas near pulmonary vessels prior to bone marrow recovery.25 In most cases of hemoptysis, the bleeding eventually stops. In a patient with known fungal disease experiencing recurrent episodes of hemoptysis, the lesion should be excised. It is becoming more common to medically treat mild hemoptysis from pulmonary aspergillosis because many of the cavities seal off and form linear scars. If an undiagnosed malignancy is found to be the cause of the hemoptysis, surgical resection is indicated when possible. Initiating chemotherapy or radiation therapy can also improve symptoms rapidly.
Pneumothorax and Pneumomediastinum
Etiology and Evaluation
A pneumothorax is air in the pleural space; pneumomediastinum is air in the mediastinum, often with subcutaneous emphysema. A tension pneumothorax arises when inspired air accumulates in the pleural space and is not expelled due to a one-way valve effect. A tension pneumothorax will eventually cause mediastinal shift and circulatory collapse.
Pneumothorax or pneumomediastinum is a rare presenting symptom of an undiagnosed malignancy such as pleuropulmonary blastoma.28 They are more likely due to infection, chemotherapy-induced emesis, postoperative complication, esophageal perforation, recurrent or metastatic disease, pulmonary fibrosis from radiation or bleomycin, pulmonary histiocytosis, or idiopathic causes.
Patients present with cough, dyspnea, or pleuritic chest pain, but often a pneumothorax is found incidentally on CXR. Examination may reveal decreased breath sounds on the affected side, tachycardia, tachypnea, shifting of the trachea, hyperresonance to percussion, or subcutaneous emphysema. A CXR may show a collapsed lung. In pneumomediastinum, air can be seen tracking through the soft tissue and under the skin. Shifting of the cardiac shadow and trachea indicates a tension pneumothorax that must be relieved immediately. Pulse oximetry or an arterial blood gas can be used to assess oxygenation. CT with contrast esophagography can rule out esophageal perforation as a cause of pneumomediastinum.
Therapy
The patient should be placed on 100% oxygen immediately. If the lesion is small, oxygen may suffice. If the lesion is large or a tension pneumothorax or pneumomediastinum is present, the excess pleural or mediastinal air must be evacuated.29 Needle thoracentesis can be performed acutely, but a chest tube should be placed for long-term evacuation.29 Tension pneumomediastinum can be relieved using a subxiphoid incision and placement of a chest tube retrosternally. In the case of recurrent pneumothoraces, pleurodesis with mechanical abrasion or a chemical agent (see Pleural and Pericardial Effusions) can prevent recurrence. Treatment of the underlying problem, such as antibiotics for infection or steroids for pulmonary fibrosis, should be started as soon as the patient is stabilized.
GASTROINTESTINAL EMERGENCIES
A very common complaint in cancer patients is abdominal pain, which can reflect issues as simple as treatment-induced nausea and constipation to an acute abdomen requiring urgent medical care. The differential diagnosis of abdominal pain is lengthy in this population, but reviewing the history and the concomitant symptoms (fever, diarrhea, bleeding), as well as judicious imaging, can readily determine the cause. This section will focus on GI emergencies, but symptomatic management can be found in Chapter 43.
Etiology and Pathogenesis
A child with cancer can develop an acute abdomen for the same reasons as any other child, but the differential diagnosis must be broadened to include issues related to the underlying malignancy and its treatment. Abdominal processes that are localized in healthy children may be generalized in the neutropenic or immunosuppressed child. GI emergencies in the child with cancer arise because of hemorrhage, mechanical obstruction, perforation, infection, and inflammation (Table 38.2). Unlike adults, most GI emergencies in children are secondary to cancer treatment rather than the underlying cancer itself. Hemorrhage may result from thrombocytopenia, coagulopathy, mucosal ulceration, or abnormal vessels within the tumor. Obstruction results from compression of a GI lumen by a tumor or abscess or from medication-induced ileus. Perforation can result from unresolved obstruction, localized ulceration, and segmental necrosis. Any part of the GI tract may become inflamed, causing esophagitis, gastritis, enterocolitis, typhlitis, and hepatitis. A patient’s inflammatory response may be blunted by neutropenia, and wound healing may be slow because of the effects of malnutrition and chemotherapy, notably corticosteroids, on fibroblast proliferation and collagen production. Massive acute hepatic enlargement can result from tumor infiltration by neuroblastoma, hemophagocytic lymphohistiocytosis (HLH), or leukemia or an acute hepatic hemorrhage by a liver primary or metastases.30 Veno-occlusive disease (VOD) can cause rapid liver failure, generally in the post-bone marrow transplant (BMT) setting.31
TABLE 38.2 Differential Diagnosis of Abdominal Pain in Pediatric Cancer Patients | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Evaluation
Pain is the principal symptom of an acute abdominal process, no matter how compromised the patient. It is important to determine the location, quality, and timing of pain in relation to the cancer location, recent medications, and surgical history. Changes in vital signs, including fever, hypertension or hypotension, and tachycardia; the presence of blood in vomitus or stool; abdominal distention; and absence of flatus or stool are nonspecific indications of an acute abdomen.
On observation, one should note whether the child lies still, is willing to move, or winces with movement or motion of the bed. Examination may also reveal abdominal distension, asymmetry, and surgical scars. Compressing the head of the stethoscope gently into the abdomen during auscultation can reveal guarding or tenderness. Auscultation can help differentiate ileus from obstruction. Inspection of the oropharyngeal and perirectal mucosa may provide insight into the state of the esophageal or gastric mucosa. Pain with defecation or detected on perineal examination may be the result of generalized serositis, mucositis, or abscess.
Laboratory and Diagnostic Studies
Selective use of laboratory and radiographic studies may differentiate the child who requires immediate surgical intervention from one who will respond to medical management. Serial CBCs can help monitor hemorrhage and reveal neutropenia that interferes with pus and abscess formation. Persistently positive blood cultures despite treatment with appropriate antibiotics may indicate the presence of an abscess or necrotic bowel. Electrolyte changes may reveal metabolic deficits from fluid shifts before they are clinically apparent. Abdominal paracentesis, diagnostic lavage, and laparoscopy have been replaced by noninvasive imaging modalities.9,32
Diagnostic radiologic studies begin with supine, erect, and left lateral decubitus abdominal radiographs. Obstruction (especially high small-bowel obstruction), pneumatosis intestinalis, and intra-abdominal free air can be diagnosed by these studies. In a large retrospective study, the presence of peritoneal signs on examination and pneumatosis intestinalis on radiograph were highly associated with the presence of an acute surgical process in children receiving chemotherapy.33 A CXR may reveal pneumonia as the etiology of upper quadrant abdominal pain. Direct examination with endoscopy or colonoscopy has replaced contrast studies in the evaluation of the GI tract in both compromised and healthy children.9,32 Abdominal US, CT, positron emission tomography (PET), and magnetic resonance imaging (MRI) may all be used to locate and characterize mass lesions, peritoneal fluid, and abnormal bowel wall thickening.
Gastrointestinal Hemorrhage
Upper Gastrointestinal Hemorrhage
Hematemesis, melena, and occult fecal blood often consists of blood swallowed from epistaxis or mucositis/esophagitis, but occasionally may signify upper GI hemorrhage. Children with cancer taking high-dose or prolonged corticosteroids, those with increased intracranial pressure (ICP), and those who underwent high-dose chemotherapy are prone to develop esophagitis, gastritis, and even gastric ulcers. Prophylaxis with oral antacids, H2 blockers, or proton-pump inhibitors (PPIs) should be considered for all at-risk children. Chemotherapy-induced emesis can cause Mallory-Weiss tears, which are mucosal lacerations at the gastroesophageal junction resulting from large gradients between the intragastric and intrathoracic pressure during emesis.34,35,36
Medical management of acute bleeding consists of initiating intravenous PPIs such as omeprazole or pantoprazole, and correction of thrombocytopenia and coagulation abnormalities.34,36 Self-limited hematemesis or coffee-ground emesis can be treated expectantly, whereas brisk bleeding often requires volume resuscitation with fluids and packed red blood cells and correction of coagulopathy with platelets and fresh frozen plasma (FFP). Passage of a large-bore nasogastric tube and gastric lavage can help differentiate an active bleed (bright red blood) from coffee-ground gastric contents. Continued bright red blood despite repeated lavage is highly suggestive of severe bleeding.34,36 Endoscopy is indicated for uncontrolled or recurrent bleeding, and hemostasis may be achieved with electrocautery. Surgery for persistent bleeding must be individualized but most often entails resection and anastamosis or oversewing of an ulcer.
Esophageal Varices
Esophageal varices are very rare in pediatric patients and can develop as a consequence of long-term portal hypertension, fibrosis, cholangitis, VOD, and cirrhosis. Variceal bleeding is brisk, and patients may have associated hematemesis and melena. A CBC, PT (INR), aPTT, and blood type and cross match should be obtained immediately. Initial management includes elevation of the head of the bed to 30 to 45 degrees, judicious volume expansion with normal saline or Ringer’s lactate, and correction of anemia. Platelets should be transfused for thrombocytopenia and FFP given if there is evidence of a coagulopathy or if multiple units of red blood cells are required. It is recommended that patients receive broad-spectrum antibiotics, as bleeding varices may be a sign of sepsis in a patient with portal hypertension and to reduce infectious complications.36 Lavage can be initiated as described above. Urgent management is indicated for persistence of bright red blood in the lavage fluid. Somatostatin (Octreotide) therapy should be initiated and continued for several days as it decreases portal pressure. If bleeding persists, endoscopy with endoscopic variceal band ligation or sclerotherapy is indicated, preferably performed within hours of the onset of bleeding. Refractory bleeding is an indication for a transjugular intrahepatic portosystemic shunt procedure. Balloon tamponade, under direct observation with a Sengstaken-Blakemore tube or Linton tube, may control refractory bleeding but is associated with considerable morbidity and mortality and should not be used for more than 24 hours.36
Lower Gastrointestinal Hemorrhage
Infections with Clostridium difficile, cryptosporidium, and fungi, and typhlitis/neutropenic enterocolitis are associated with bloody stools. Typhlitis is the most common cause of lower GI bleeding in adults with acute leukemia and is discussed in more detail below.35 Intussusception due to tumor, surgery, or an abscess can cause pain, intermittent bleeding, or currant jelly stools.37 Most major lower GI hemorrhage in the pediatric cancer patient is multifactorial, for example, C. difficile enterocolitis in a child with thrombocytopenia and coagulopathy. Hemorrhoids and anal fissures can cause mild bleeding, often reported as blood in the toilet bowl or on the toilet paper. Management is directed at each of the underlying causes.
Gastrointestinal Obstruction
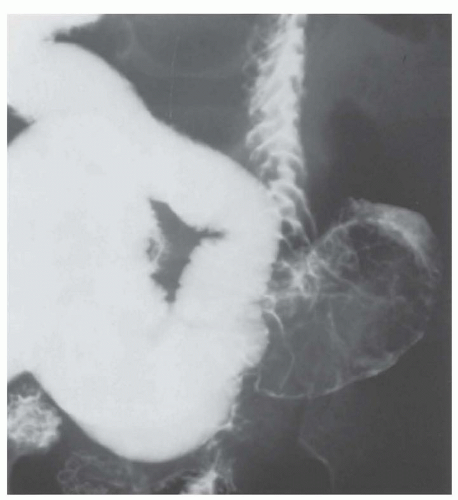
Although bowel obstruction from primary cancer is rare in children, it can be seen with de novo or refractory sarcoma (especially desmoplastic round cell sarcoma), abdominal lymphoma, bulky renal/adrenal tumors, ovarian and colon carcinoma, and presacral teratoma. Obstruction from small-bowel intussusception (Fig. 38.4) is the presentation of 17% to 25% of abdominal Burkitt lymphomas.37 The differential diagnosis of bowel obstruction in the child who has had adjuvant therapy or previous abdominal surgery includes obstipation or paralytic ileus induced by vinca alkaloids or narcotics, adhesions or strictures, and intussusception.9,32 Abdominal compartment syndrome, defined as elevated intra-abdominal pressure (≥20 mm Hg) associated with respiratory compromise and renal insufficiency, can be seen in patients with bulky abdominal tumors at diagnosis or as a postoperative complication.38
History, physical examination, and plain radiographs that document obstipation can help differentiate narcotic or
vincristine-related ileus from a surgical abdomen. CT scan with oral contrast can delineate soft tissue masses, air in the bowel wall, and sometimes reduce intussusception.32 Small-bowel obstruction is initially managed by making a patient NPO and placing a nasogastric tube for decompression. Evaluation for the underlying etiology should proceed as described in laboratory and diagnostic studies. Intussusception is often diagnosed and temporarily reduced by air-contrast or barium enema, but surgical reduction is generally necessary for intussusception caused by leading-edge tumors.37,39
vincristine-related ileus from a surgical abdomen. CT scan with oral contrast can delineate soft tissue masses, air in the bowel wall, and sometimes reduce intussusception.32 Small-bowel obstruction is initially managed by making a patient NPO and placing a nasogastric tube for decompression. Evaluation for the underlying etiology should proceed as described in laboratory and diagnostic studies. Intussusception is often diagnosed and temporarily reduced by air-contrast or barium enema, but surgical reduction is generally necessary for intussusception caused by leading-edge tumors.37,39
Gastrointestinal Perforation
Perforation may be the outcome of unresolved obstruction, ulcers, or gastritis unresponsive to medical therapy, infections such as typhlitis, or erosion by the primary tumor.9,32 In the case of abdominal Burkitt lymphoma, perforation may occur at presentation, during therapy, or after clinical improvement in association with necrosis of the underlying tumor.40 Upright abdominal radiographs may reveal air under the diaphragm, tracking into the liver, or along the flank on a decubitus view. CT scan may also show free air but is better at delineating pneumatosis intestinalis, infectious abscess, extravasation of oral contrast, and an obstructing soft tissue lesion.9,32 Perforations are a surgical emergency and are managed either by resection of the affected area and secondary closure or by primary reanastomosis, along with broad-spectrum antibiotics.9,32,40
Gastrointestinal Infection and Inflammation
Typhlitis, also referred to as neutropenic enterocolitis, is a necrotizing colitis typically localized to the terminal ileum and cecum, but can occur anywhere in the colon and occasionally in the small bowel. Traditionally, it was defined as the triad of neutropenia, abdominal pain, and fever, primarily seen in patients with acute leukemia. Improved imaging, revealing bowel wall thickening and pneumatosis, has enabled diagnosis of typhlitis in both neutropenic and non-neutropenic patients, and in children with solid tumors. Symptoms in order of frequency include abdominal pain (90%), fever (84%), diarrhea (72%), and, less frequently, nausea/vomiting, and GI bleed. The incidence of typhlitis in children with cancer is reported at 1.4% to 11.6%, and 65% to 75% of cases are in patients with hematologic malignancies.41,42,43,44
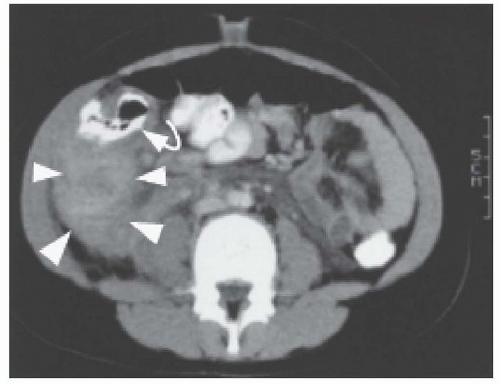
Typhlitis usually occurs within 2 weeks of cytotoxic chemotherapy or steroids and tends to occur as the ANC is nadiring.41,42,43 Other risk factors for typhlitis include age >10 years, being post-BMT, and visible mucositis. The cause of typhlitis is unclear but is believed to result from a combination of mucositis, bacterial overgrowth leading to bowel wall edema, and impaired vascular blood flow to the intestinal wall. This can lead to worsening ulceration, necrosis, and perforation.41,42,43,45 Gram-negative bacteria, such as Pseudomonas species, Escherichia coli, and Clostridium species, are the most common bacterial pathogens, but Staphylococcus, Streptococcus, and Enterococcus species have been identified.41,45,46,47,48 Fungus, including Candida and Aspergillus species, has been identified as less frequent pathogens.43,44 Radiographic studies may demonstrate thickening of the bowel wall (>0.3 mm), free air, or pneumatosis intestinalis.41 In a retrospective review of 24 children with typhlitis, Sloas et al.45 found that CT scan (Fig. 38.5) and US were more sensitive than plain radiographs; false-negative rates were 15% for CT, 23% for US, and 48% for plain radiographs. US is reported to be equivalent to CT in detecting typhlitis and appendicitis and eliminates radiation exposure.41 Patients with bowel wall thickening greater than 1 cm on US are more likely to have a longer duration of symptoms and a worse outcome.
In the past, the mortality rate from typhlitis ranged from 20% to almost 100% with either surgical or medical treatment, but now stands at 0% to 8.3%, primarily with medical treatment alone.41,42,45 Surgical intervention in typhlitis should be reserved for persistent GI bleeding despite resolution of thrombocytopenia and correction of clotting abnormalities, evidence of free air or perforation, necrotic bowel causing sepsis, obstruction, or failure of medical management.43,44 In two recent reports, only 4% to 8% of patients with typhlitis ultimately required a surgical intervention.41,42 Pneumatosis and localized peritoneal signs are not sufficient to warrant surgical exploration in the absence of one or more of the above indications. Using these criteria, most patients can be managed medically with broad-spectrum antibiotics to cover gram-negative pathogens, GI anaerobes (metronidazole or a carbapenem), and fungi.
Typhlitis and appendicitis have very similar presentations in children with cancer. Appendicitis also presents with abdominal
pain, but typhlitis is more frequently associated with fever, diarrhea, and prolonged neutropenia.49 Incidence rates of appendicitis range from 0.5% to 1.5% and is primarily seen in children with leukemia or lymphoma.49 Despite the sensitive imaging modalities of US and CT, one-third of oncology patients with appendicitis have a delay in diagnosis due to blunted inflammatory response and a broad differential of abdominal pain.49,50 Case reports exist of appendicitis being treated successfully with conservative medical management alone.51
pain, but typhlitis is more frequently associated with fever, diarrhea, and prolonged neutropenia.49 Incidence rates of appendicitis range from 0.5% to 1.5% and is primarily seen in children with leukemia or lymphoma.49 Despite the sensitive imaging modalities of US and CT, one-third of oncology patients with appendicitis have a delay in diagnosis due to blunted inflammatory response and a broad differential of abdominal pain.49,50 Case reports exist of appendicitis being treated successfully with conservative medical management alone.51
Patients who do not have typhlitis but have signs and symptoms of enterocolitis may have transverse colitis, intussusception, or antibiotic-related pseudomembranous or clostridial enterocolitis. C. difficile colitis is now the major cause of nosocomial diarrhea in the United States and Western Europe.48 Although use of second- and third-generation cephalosporins, clindamycin, ampicillin, and amoxicillin is associated with the highest risk of C. difficile colitis, even a single dose of almost any antibiotic can predispose a patient to C. difficile colitis. The specific therapy for C. difficile colitis is oral metronidazole or oral vancomycin.48 Infection with C. septicum is also a diagnostic consideration in neutropenic patients, and may present without significant fever, but can progress rapidly.52 Any suspected abdominal infection in neutropenic patients merits urgent antibiotic coverage.
Perirectal Abscess
Anorectal pain, tenderness, and discomfort with bowel movements may indicate a perirectal abscess or fistula. Perirectal abscesses occur in patients with neutropenia, especially those with acute myelogenous leukemia (AML).44 In a neutropenic patient, the only physical finding may be tender, brawny woody edema with a dense cellulitic reaction. Most abscesses are caused by a mixture of aerobes (including staphylococci, E. coli, Pseudomonas, and streptococci) and fecal anaerobes. Initial therapy includes antibiotics to cover aerobic and anaerobic gram-negative and gram-positive bacteria. Sitz baths may relieve pain. If the abscess or induration is well circumscribed or progresses, the lesion may need to be incised and drained, but early aggressive medical management often eliminates the need for surgical intervention, particularly while the patient is neutropenic.
Gastrointestinal Organ Dysfunction
Cholecystitis and Biliary Obstruction
Inflammation of the liver and biliary tract can present as localized right upper quadrant pain or jaundice. The most common cause of hyperbilirubinemia, often asymptomatic, is temporary hepatic toxicity from chemotherapy. Acute cholecystitis, particularly acute acalculous cholecystitis, occurs in children who are septic, stressed, and volume depleted. Biliary obstruction as a result of primary tumor is rare: lymphoma and neuroblastoma occasionally block biliary flow, and rhabdomyosarcoma of the common duct occurs.
Ultrasonography or CT scan can differentiate calculous from acalculous cholecystitis. Hydration, broad-spectrum antibiotics, and nasogastric decompression usually treat the acute cholecystitis. Antibiotics should cover gram-negative bacilli and, in the neutropenic patient, fungi. Endoscopic retrograde cholangiopancreatography or combined endoscopic-cutaneous decompression is successful in most patients, and stent placement may provide effective palliation for up to 6 months.
Acute Massive Hepatomegaly
Massive hepatomegaly may complicate stage IV-S neuroblastoma or HLH in the neonate and may be fatal. Unless life-threatening respiratory embarrassment is present, supportive care and observation are sufficient until the disease is treated. If hepatomegaly compromises respiratory function, therapeutic options include standard chemotherapy and radiation for neuroblastoma, or appropriate HLH-directed therapy.30 Radiation therapy involves lateral portals with the child supine and fields encompassing most of the liver, but sparing the ovaries and kidneys, and extending from the dome to the posterior portion of the right hepatic lobe. A midplane dose of 150 cGy given on 3 successive days has been sufficient to ameliorate life-threatening hepatomegaly resistant to chemotherapy.30
Veno-occlusive Disease
VOD, also referred to as hepatic sinusoidal obstruction syndrome, consists of rapid and often massive hepatic enlargement with resultant right upper quadrant pain, liver tenderness, jaundice, weight gain, and ascites. VOD is most frequently a complication of cytoreductive therapy for BMT and can range from mild to severe.31,53 Preexisting hepatic disease, radiation, allogeneic transplant, use of busulfan with our without CPM, and younger age have all been identified as risk factors for developing VOD in BMT.31,53 Mild-to-moderate reversible VOD has also occurred following vincristine and actinomycin-D with or without CPM therapy for rhabdomyosarcoma and Wilms tumor.54 Those younger than 36 months of age were found to be at greatest risk for the development of hepatopathy after treatment with vincristine, actinomycin-D, and CPM for rhabdomyosarcoma, with an incidence of 5%.54 Withholding vincristine or actinomycin-D generally leads to symptomatic improvement. Gemtuzumab therapy for AML is associated with a 15% to 24% incidence of VOD, primarily developing days to weeks into BMT.55
In both the Children’s Oncology Group (COG) and United Kingdom pediatric ALL trials that randomized 6-mercaptopurine with thioguanine in maintenance, thioguanine was associated with a significantly increased incidence of VOD during treatment, with a male predominance.56,57 The rate of VOD in the thioguanine-treated patients was 10% to 15% and higher if patients with evidence of portal hypertension such as splenomegaly and thrombocytopenia were included. Although symptoms resolved in most patients within weeks of stopping antimetabolites, more than 10% of affected patients had persistent splenomegaly and thrombocytopenia, as well as evidence of varices, abnormal liver biopsies, and portal hypertension requiring close follow-up by a gastroenterologist.56,57,58
Treatment of VOD is primarily supportive in the nontransplant setting.54 Historically, children who developed irreversible VOD post-BMT had a mortality rate approaching 100%, but use of defibrotide (a fibrinolytic, antithrombotic, and anti-ischemic agent) has been associated with complete response rates between 29% and 46% and a 42% to 62% survival at 100 days post-BMT.31,53,59,60 Newer data also support the use of prophylactic defibrotide (6.25 mg/kg) IV 4 times daily in pediatric patients at high risk of developing VOD in BMT.31
Pancreatitis
When vomiting and abdominal pain occur in a child receiving asparaginase or corticosteroids or in a child with ICP, pancreatitis should be included in the differential diagnosis.61,62 The reported incidence of asparaginase-associated pancreatitis ranges from 1% to 18%, tending to occur early in ALL/NHL therapy. Studies have shown that pancreatitis is associated with concomitant steroid use and older age.61,62 An elevated pancreatic amylase and lipase usually make the diagnosis, and imaging may only be necessary for severe or persistent pain. An US or CT scan with contrast may show a phlegmon as a diffuse homogenous area, and pancreatic pseudocysts may appear as loculations of various densities within the pancreas.50
The majority of patients with clinically symptomatic pancreatitis require inpatient admission, complete bowel rest, and intravenous narcotics for pain management.63 Parenteral nutrition may be necessary. Placement of a nasogastric tube is indicated for intractable emesis or an ileus. Antibiotics should be started for evidence of pancreatic necrosis, abscess, or phlegmon, and should
include gram-negative and anaerobic coverage. Octreotide, a synthetic somatostatin analog, may ameliorate the clinical course of pancreatitis in children.64 Most cases of pancreatitis are self-limited and can be treated medically. Indications for surgical drainage of a pancreatic abscess or pseudocyst depend on the child’s condition and the evolution of the process on sequential US or CT examinations. Necrotizing pancreatitis and large pseudocysts causing severe pain or obstruction often require urgent surgical drainage. Percutaneous CT-guided drainage by an interventional radiologist may also be effective.65
include gram-negative and anaerobic coverage. Octreotide, a synthetic somatostatin analog, may ameliorate the clinical course of pancreatitis in children.64 Most cases of pancreatitis are self-limited and can be treated medically. Indications for surgical drainage of a pancreatic abscess or pseudocyst depend on the child’s condition and the evolution of the process on sequential US or CT examinations. Necrotizing pancreatitis and large pseudocysts causing severe pain or obstruction often require urgent surgical drainage. Percutaneous CT-guided drainage by an interventional radiologist may also be effective.65
GENITOURINARY EMERGENCIES
Renal complications in children with malignancies can be divided into primary complications caused by the tumor itself, such as urinary tract obstruction and renal vein thrombosis, and those that are secondary to the therapy, such as hemorrhagic cystitis, acute renal failure, or hypertension.
Oliguria or Anuria
When a child has reduced or absent urine output, it is necessary to determine whether the cause is prerenal, renal, or postrenal. In children receiving cancer treatment, septic shock is the most common prerenal cause of reduced urine output. Chemotherapy-induced emesis, profuse infectious diarrhea, negligible oral intake, and metabolic abnormalities all can cause intravascular depletion and oliguria.66 Bulky abdominal or pelvic tumors, including retroperitoneal sarcomas and lymphomas, germ cell tumors, ovarian tumors, and adrenal or celiac axis neuroblastoma, may compress or obstruct the ureters or bladder, causing postrenal failure, as illustrated in Figure 38.6.66,67 Abdominal compartment syndrome from bulky tumors or postoperative complications can also result in renal insufficiency and oliguria.38 Intravesicular blood clots from hemorrhagic cystitis can lead to ureteral or bladder outlet obstruction.68 Many chemotherapy agents (e.g., methotrexate, cisplatin), as well as supportive care medications such as antibiotics, antifungals, antivirals, and IV contrast for imaging can cause renal insufficiency, especially when used in combination.66 Some of the new molecular agents that target VEGF such as tyrosine kinase inhibitors and bevacizumab have been associated with renal toxicity including proteinuria, hypertension, nephrotic syndrome, and acute kidney injury.69 Narcotics, vincristine, phenothiazines, and herpes zoster can affect the sacral nerves and cause urinary retention, which is sometimes responsive to nalbuphine.
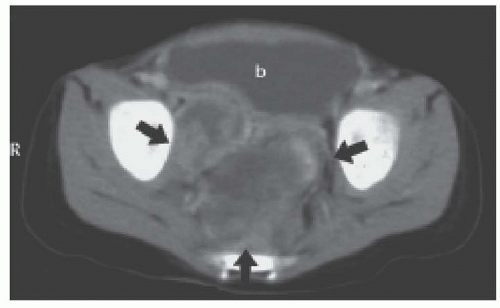
In a child presenting with a new abdominal or pelvic mass, one should inquire about urinary or bowel dysfunction and send blood for blood urea nitrogen (BUN), creatinine (Cr), and electrolytes. CT or US of the abdomen and pelvis can reveal a large mass, renomegaly, and delayed filling of the bladder. If there is no evidence of impending renal failure, contrast may be used. Postrenal oliguria from compression or obstruction must be differentiated from tumor lysis syndrome (TLS), as both can present with elevated uric acid, potassium, and phosphorus, although a very elevated BUN and Cr are more consistent with postrenal obstruction.
Therapy
Vigorous hydration quickly corrects oliguria in patients with prerenal failure but should be avoided in postrenal failure. In most cases of obstructive uropathy with resultant hydronephrosis, decompression of the obstructed kidney by urgent placement of a urinary catheter, percutaneous nephrostomy tube, or ureteral stents will lead to rapid improvement.67 Treatment of the underlying tumor with surgery, radiation, or chemotherapy will ultimately relieve the obstruction. In renal insufficiency, nephrotoxic drugs should be used cautiously with drug level monitoring and avoided in combination when possible.
Hemorrhagic Cystitis
Etiology and Evaluation
Signs and symptoms of hemorrhagic cystitis are hematuria or clots in the urine caused by bleeding and inflammation of the bladder, leading to dysuria, urgency, and frequency. Substantial blood loss and urinary obstruction can result. Therapy with CPM and ifosfamide (IFOS) is the most common cause of hemorrhagic cystitis, which can occur hours to months after administration.68 Acrolein, their principal metabolite, is toxic when it precipitates in the bladder.68 Recent data show that hemorrhagic cystitis in most BMT patients is actually associated with BK virus, which can be found (by BK DNA PCR testing) in up to 80% of pediatric patients with hemorrhagic cystitis.68,70 BK virus-associated nephropathy includes both hemorrhagic and nonhemorrhagic cystitis, interstitial nephritis, and ureteral stenosis. Other viral etiologies of hemorrhagic cystitis include adenovirus, cytomegalovirus, and JC virus.68,70,71 In patients with prolonged hemorrhagic cystitis presumed secondary to chemotherapy, radiation therapy, or post-BMT, viral DNA PCR studies should be done.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree