INTRODUCTION
Oncologic emergencies can result from either the cancer or its treatment. Cancer patients often have immunologic, metabolic, and hematologic defects, which can lead to complex emergency conditions when they present to an emergency center. In addition, emergencies resulting from comorbid conditions also occur in cancer patients. It is important for practitioners who treat patients with cancer to be aware of the various oncologic emergencies so that they can be recognized and treated promptly. This chapter discusses many of these emergencies, including their signs and symptoms, causes, and management.
NEUROLOGIC EMERGENCIES
Spinal cord compression is a serious complication of cancer progression, affecting about 2.5% of cancer patients overall (1). It is not immediately life-threatening unless it involves the first three cervical vertebrae, but involvement in the rest of the spine leads to significant morbidity (2). The spinal cord is compressed at the thoracic vertebrae in 70% of patients, cervical vertebrae in 10% of patients, and lumbar vertebrae in 20% of patients. In 10% to 38% of cases, spinal cord compression occurs at multiple levels (3). Such compression is predominantly due to metastatic tumors, with lung, breast, and prostate cancer comprising 50% of these. Other tumors that commonly metastasize to the spine are multiple myeloma, renal cell carcinoma, melanoma, lymphoma, sarcoma, and gastrointestinal (GI) cancers. The mechanisms by which tumors can appear in the spine are hematogenous spread of tumor cells to the vertebral bodies, metastasis of primary lesions to the posterior spinal elements, and direct extension of paraspinal tumors. Spinal cord compression is caused by epidural metastases in 75% of cases and bony collapse in 25% of cases (4).
The most common presentation of spinal cord compression is back pain, occurring in over 90% of patients. Depending on the location of the tumor in the spinal canal, the pain can be unilateral or bilateral following dermatomal patterns. Patients typically report that their pain is worse when they are supine and better when they are upright. Ataxia due to compression of the spinocerebellar tracts can be confused with cerebellar metastasis, overmedication with analgesics, or other disorders. Metastasis to the spinal cord can precede spinal cord compression by weeks or months. The patient may also note sensory symptoms, including numbness or tingling in the toes, which can progress proximally. Preexisting peripheral neuropathy must be differentiated from spinal cord compression and acute worsening of existing symptoms or experienced new numbness or tingling. Motor symptoms are the second most common complaint after pain; difficulty walking, buckling under of the legs, and a feeling of heaviness in the legs are all frequent symptoms. The last symptoms to appear are autonomic symptoms, such as urinary retention and constipation. Autonomic symptoms are late findings in spinal cord compression and must be distinguished from the effects of chemotherapy, pain medicines, and antihistamines. It is important to remember that the patient may present with intractable pain only, so a high level of suspicion for spinal cord compression is important in treating cancer patients.
The physical examination usually reveals tenderness to percussion over the affected level of the spine, but the spine might not be tender if there is no bone involvement. Other possible findings are urinary retention, decreased rectal sphincter tone, and muscle weakness. The patient might have pain at a referred site; for instance, patients with L1 compression might have pain in the sacroiliac area. Sensory changes are more difficult to diagnose than motor deficits and can either precede or accompany motor effects. The patient might have decreased sensation in the lower extremities, which may ascend to the level of spinal cord involvement with dorsal column deficits, including loss of light touch sensation, proprioception, and position sense. When the cauda equina is compressed, the sensory changes are dermatomal, with loss of sensation in the perineal area, the posterior thigh, or lateral leg.
The differential diagnosis of spinal cord compression includes osteoarthritis, degenerative disk disease, spinal abscess, hematoma/bleeding, hemangioma, chordoma, meningioma, and neurofibroma. A standard x-ray is generally ordered first to analyze the area of the spine within which compression is suspected. However, simple roentgenography yields false-negative results in 10% to 17% of cases, in part because approximately 30% to 50% of the bone must be destroyed before bony lesions can be seen on x-ray films (5). Magnetic resonance imaging (MRI) is the imaging technique of choice today for suspected spinal cord compression (Fig. 53-1).
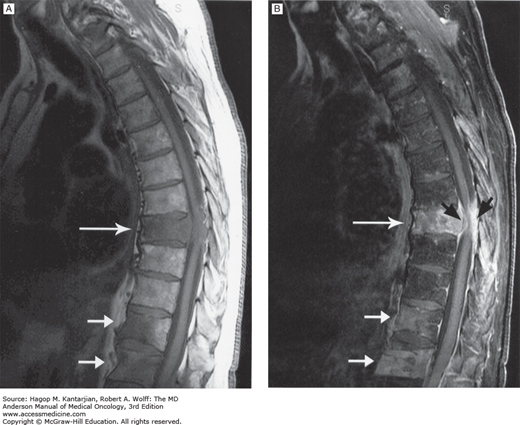
FIGURE 53-1
A. Precontrast T1-weighted magnetic resonance image (MRI) of thoracic cord compression at the T8 level produced by an epidural tumor from vertebral body metastasis (large arrow). Smaller arrows point to other sites of bony metastasis. The patient is a 67-year-old man with melanoma and back pain. B. Postcontrast T1-weighted MRI of the same patient. The epidural tumor is visualized better with contrast (black arrows). (Used with permission from Dr. Ashok Kumar, MD Anderson Cancer Center.)
For patients with suspected spinal cord compression, physicians should consider imaging the entire spine because spinal epidural disease is often multifocal. Findings for the whole spine can help the physician optimize the type and extent of therapy needed. For any patient with rapidly progressive neurologic symptoms, diagnostic imaging should be performed on an emergency basis. Magnetic resonance imaging of the spine is the diagnostic study of choice. Gadolinium enhancement will be helpful in detecting other causes of neurologic symptoms such as epidural abscess or leptomeningeal metastasis. Patients who are not able to undergo MRI (eg, the presence of paramagnetic cerebral aneurysm clips, or cardiac pacemakers) can undergo computed tomography (CT) myelogram.
Clinical guidelines for diagnosis and management of spinal cord compression are available (6,7). Corticosteroid is a temporizing measure to stabilize or even improve neurologic function until definitive treatment. Conventionally, dexamethasone is initially given at 10 to 100 mg intravenously and then 4 to 24 mg every 4 to 6 hours (8). The duration of therapy with high-dose glucocorticoids should be minimized to prevent complications of steroid use.
Surgery is indicated for recurrent or progressive disease at an area with previous maximal radiotherapy, spinal mechanical instability, an unknown tissue diagnosis of malignancy, or for compression of the spinal cord by bony structure/fragment (6,7). Currently, anterior decompression with spinal stabilization is the surgery of choice, allowing removal of the affected vertebral body and stabilization above and below the vertebrae by metal hardware. Surgical resection followed by radiotherapy may improve ambulation ability and survival better than radiotherapy alone (9). Benefit from decompressive surgery is evident in ambulatory patients with poor prognostic factors for radiotherapy and in paralyzed patients with a single spinal area of compression, paraplegia less than 48 hours, non-radiosensitive tumors, and an expected survival of more than 3 months (10). If surgery is not indicated, radiation therapy can be used for radiosensitive tumors; the most common dosage is 3,000 cGy delivered in 10 fractions (3). The incidence of myelopathy, which can occur as a complication of radiation therapy, increases with increasing total dosage of therapy and can appear from months to several years after such therapy is given. Palliative radiotherapy is recommended for those with paraplegia longer than 48 hours, expected to live for fewer than 3 months, unable to tolerate surgery, and with multiple areas of compression. An ambulatory patient with a stable spine may be considered for radiation treatment (8). Chemotherapy is occasionally used for chemotherapy-sensitive tumors, such as Hodgkin disease, neuroblastoma, non-Hodgkin lymphoma, germ cell tumors, and breast cancer.
One of the most important prognostic factors at diagnosis is the patient’s neurologic function. Of patients who are ambulatory at the time of presentation, approximately three-fourths will be able to regain their strength with treatment. By contrast, only a small percentage of patients who are paralyzed at the time of presentation are likely to walk again. This difference illustrates why it is imperative to diagnose spinal cord compression at an early stage. A scoring system based on tumor type, interval between tumor diagnosis and spinal cord compression, other bone or visceral metastases, ambulatory status, and duration of paralysis can estimate survival (11). The median overall survival following the first episode of spinal cord compression is about 3 months (1).
Increased intracranial pressure in cancer patients is commonly due to hemorrhage (from thrombocytopenia or tumor bleeding), brain metastasis with vasogenic edema and mass effect, or hydrocephalus due to obstruction of the flow of cerebrospinal fluid (CSF). Increased intracranial pressure can also be caused by tumor treatments, such as radiation therapy and surgery. The normal CSF pressure is less than 10 mm Hg. As intracranial pressure increases, herniation syndromes may develop, including uncal, central, and tonsillar herniation. Uncal herniation is caused by unilateral supratentorial lesions that push brain tissue through the tentorial notch. Signs and symptoms include ipsilateral pupil dilation, decreased consciousness, and hemiparesis, first contralateral and then ipsilateral to the mass. Central herniation involves bilateral supratentorial lesions that displace tissue symmetrically and bilaterally. Signs and symptoms of central herniation include decreased consciousness leading to coma and Cheyne-Stokes respiration, followed by central hyperventilation, midposition unreactive pupils, and posturing. Tonsillar herniation involves increased pressure in the posterior fossa, which forces the cerebellar tonsil through the foramen magnum, thereby compressing the medulla. Signs and symptoms of tonsillar herniation include decreased consciousness and respiratory abnormalities leading to apnea. Headache is the most frequent symptom reported in increased intracranial pressure. Headache is a common symptom in any patient population, but in cancer patients, the clinician must always maintain a high index of suspicion for increased intracranial pressure. Headaches due to increased intracranial pressure are typically present on waking in the morning, recur throughout the day, and are increased with Valsalva maneuver; they can be associated with nausea and vomiting, altered mental status, vision changes, seizures, or focal neurologic deficits. On physical examination, the patient might have papilledema, focal neurologic deficits, or a decreased level of consciousness.
The diagnosis of increased intracranial pressure can be ascertained from CT scans of the brain. Noncontrast CT imaging of the brain is superior to MRI in detecting acute hemorrhage (Fig. 53-2).
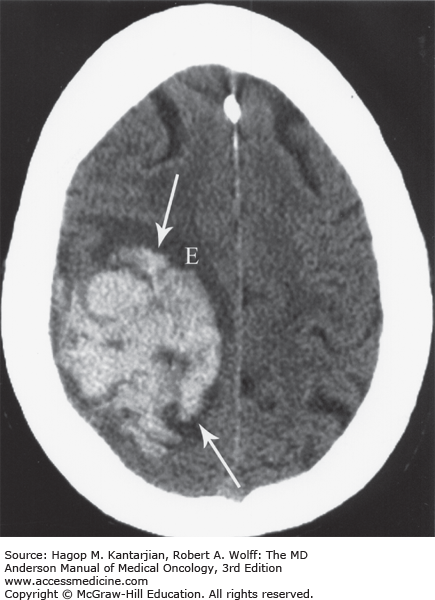
FIGURE 53-2
Acute intracranial hemorrhage within the right frontoparietal lobe (arrows) with edema (E) in a 79-year-old woman with ovarian cancer. The hemorrhage was revealed by noncontrast computed tomography imaging. This modality is superior to magnetic resonance imaging in detecting acute hemorrhage. (Used with permission from Dr. Ashok Kumar, MD Anderson Cancer Center.)
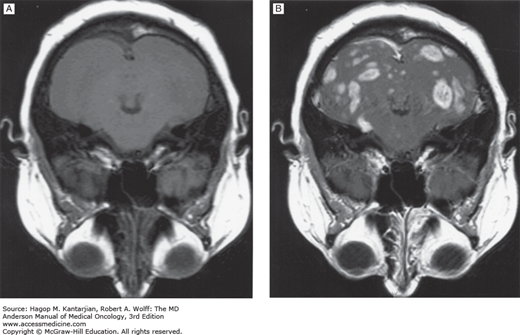
Computed tomography scans with contrast will usually reveal cerebral metastasis and occasionally leptomeningeal disease (LMD). Contrast-enhanced MRI is more sensitive than CT in revealing cerebral neoplasms and metastases as small as 3 mm (Fig. 53-3), LMD (Fig. 53-4), and early strokes (Fig. 53-5). Lumbar puncture should not be used to diagnose increased intracranial pressure, because this can lead to brain herniation.
FIGURE 53-4
A. Sagittal postcontrast T1-weighted magnetic resonance imaging (MRI) showing subarachnoid spread of melanoma metastasis to the brain in a 29-year-old man. Abnormal enhancement of the cortical sulci (large arrows) and cerebellar sulci (small arrows) is noted. B. Coronal postcontrast T1-weighted MRI in the same patient. (Used with permission from Dr. Ashok Kumar, MD Anderson Cancer Center.)
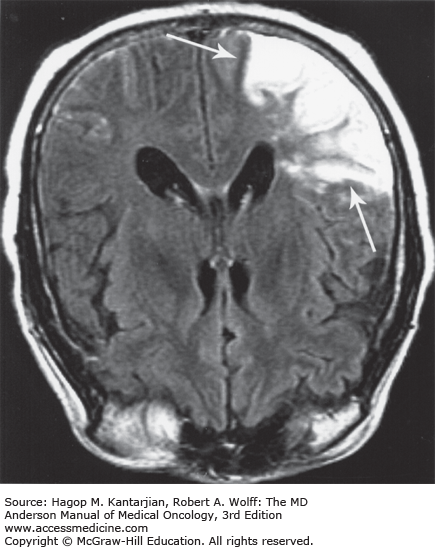
FIGURE 53-5
Acute infarction involving the territory of the right middle cerebral artery in a 58-year-old patient with renal cell carcinoma. Magnetic resonance imaging (MRI; fluid-attenuated inversion recovery [FLAIR] image) demonstrates abnormal thickening, with a T2-weighted increase in signal intensity (arrows) involving the right temporo-occipital lobe cortex and subcortical white matter. MRI is more sensitive than computed tomography in detecting early stroke. (Used with permission from Dr. Ashok Kumar, MD Anderson Cancer Center.)
The differential diagnosis of increased intracranial pressure includes bleeding, tumor edema, hydrocephalus, postradiation effects, postradiosurgery effects, brachytherapy-induced changes, benign tumor effects, subdural hematomas, meningitis, encephalitis, and abscess formation.
Brain metastases may develop in 10% to 40% of cancer patients (12). Leptomeningeal disease occurs in 5% of all patients with cancer (13). Two-thirds to three-quarters of brain metastases are recognized as multiple lesions on MRI. Lung cancer is the neoplasm that most frequently metastasizes to the brain, followed by breast cancer and melanoma. Other cancers that commonly metastasize to the brain are colorectal, kidney, prostate, testicular, and ovarian cancers and sarcomas, although any systemic cancer can metastasize to the brain. Melanomas have the highest propensity to metastasize to the brain, with up to 40% of cases behaving in this manner at some point. Tumors most commonly metastasize to the gray-white junction where the vessels are small and narrow and tumor emboli can be trapped. Eighty percent of tumors metastasize to the cerebral hemispheres, 15% to the cerebellum, and 5% to the brainstem. Pelvic tumors have an increased propensity to metastasize to the posterior fossa, possibly by means of venous drainage of these tumors through Batson plexus (14). The tumors that are most often hemorrhagic include melanoma, renal cell carcinoma, and choriocarcinoma.
The treatment for increased intracranial pressure depends on the underlying etiology (15). Infectious sources, such as meningitis, should be treated with antibiotics, and brain abscesses should be drained. Hydrocephalus should be treated with surgical shunting or ventriculostomy, and subdural hematomas should be either drained or, if small, monitored under the guidance of a neurosurgeon. Edema associated with brain tumors is initially treated with oral dexamethasone at a dosage of 16 mg/d or 4 mg every 6 hours (16). For patients with impending herniation, very large doses of intravenous (IV) dexamethasone can be used, initially 40 to 100 mg intravenously and subsequently 40 to 100 mg/d (3). Dexamethasone is the steroid of choice because of its lack of mineralocorticoid effect. Steroids may not be needed in asymptomatic brain lesions (17).
For life-threatening edema or brain herniation, emergency treatments include hyperventilation and administration of mannitol in addition to steroids (15). Hyperventilation after intubation to achieve a partial pressure of carbon dioxide of 25 to 30 mm Hg is the most rapid way to decrease intracranial pressure, but the benefit is generally short lived, and equilibration may occur within a few hours. Mannitol is a hyperosmotic agent that can shift water out of brain cells and into the vessels. The recommended dose of mannitol is a 20% to 25% solution at 0.5 to 2.0 g/kg administered intravenously over 10 to 30 minutes. Mannitol has a rapid onset of action and lasts for hours, but prolonged use can lead to hyperosmolarity and an inadvertent increase in intracranial pressure (3). Further treatment in intensive care may include IV infusion of hypertonic saline, propofol, and hypothermia (4). Neurosurgical intervention such as placement of a ventricular drain or decompressive craniectomy may be necessary if the patient has neurologic deterioration despite appropriate medical management.
Radiation therapy can be used to treat brain metastasis. The dosage for whole-brain radiation therapy (WBRT) typically ranges between 20 Gy over 1 week and 50 Gy over 4 weeks. Treatment with WBRT can increase survival in patients by 3 to 6 months relative to no treatment (16). Increased intracranial pressure should be treated before WBRT is instituted because radiotherapy can further increase pressure. Common side effects of WBRT are nausea and vomiting, alopecia, headache, hearing loss, loss of taste, and fever. Possible delayed complications of WBRT are progressive leukoencephalopathy with dementia, ataxia, apraxia, and incontinence syndrome, which can mimic normal-pressure hydrocephalus. This dreaded side effect can occur as long as 1 year after therapy, and elderly patients are more susceptible.
Surgery can be used to treat accessible brain metastases. A stereotactic biopsy can be performed for the patient with multiple brain metastases, which are then generally treated with radiation (18). Surgery is generally not indicated for patients with widespread systemic disease, poor functional status, or tumors in critical or hard-to-access locations (16). In selected patients with good functional status, even when multiple brain metastases are present, survival time is longer for patients who have all tumors removed than for those who do not. Consequently, it is common for the neurosurgeons at our institution to remove up to four metastatic lesions at a time (17). Patients with single brain metastases who underwent WBRT after surgery had longer survival than those who had surgery alone (19).
For patients with brain lesions that are not amenable to surgery, stereotactic radiosurgery can be used in single doses as high as 1,400 cGy. This approach is typically used for brain tumors less than 4 cm in diameter and has the benefit of being noninvasive and relatively fast acting (5). Brachytherapy can be used on larger tumors, but this approach requires that radioactive seeds be invasively implanted in the designated area and left for 5 or 6 days, delivering approximately 6,000 cGy to the area. Brachytherapy may cause radiation necrosis in up to 50% of patients 6 months after treatment. No treatment exists for radiation necrosis, although the symptoms may respond to corticosteroids.
Chemotherapy can be used in some patients with brain metastasis. Dexamethasone, which is thought to aid in reestablishing the blood-brain barrier, should not be used if possible, so that the selected chemotherapeutic agent(s) can reach the tumor cells. Cancers for which chemotherapy has been used include choriocarcinoma, small cell cancer of the lungs, and breast cancer (16,20,21).
Leptomeningeal disease can involve invasion of the brain, the spinal parenchyma, the nerve roots, and blood vessels of the nervous system. The cancers that most commonly result in LMD are breast and lung cancer, melanoma, non-Hodgkin lymphoma, and leukemia. Patients present with a variety of symptoms depending on the location of the leptomeninges affected, but they can include headache, altered mental status, cranial nerve palsies (in about 50% of patients), incontinence, back pain, sensory changes, seizures, isolated neurologic findings, and even a stroke-like presentation (5,21). Leptomeningeal metastases occur in 0.8% to 8% of all cases of cancer (5).
The diagnosis of LMD can be difficult. Computed tomography scans will occasionally be suggestive of LMD. Magnetic resonance imaging scanning has better sensitivity than CT for detecting LMD, including leptomeningeal enhancement, hydrocephalus, and cortical nodules. However, MRI results are not diagnostic. Inflammation of the meninges can also be found in cases of meningitis, trauma, infection, and hematoma formation. Lumbar puncture and evaluation of the CSF is the gold standard for diagnosing LMD, although multiple lumbar taps may be required to make the diagnosis because only 50% of patients will have positive cytologic evidence of LMD on the first CSF evaluation (5,22). Cerebrospinal fluid findings consistent with LMD include a high opening pressure, low glucose and high protein levels, and a mononuclear pleocytosis (5). Among patients with normal values for CSF protein, glucose, and opening pressure and cytology negative for LMD, fewer than 5% will have LMD (22).
The treatment of LMD can include chemotherapy through an implanted subcutaneous reservoir and ventricular catheter (SRVC) or through lumbar puncture instillation. Lumbar tap administration does not require placement of a catheter, but 10% to 15% of the subarachnoid space might be missed using this technique. Chemotherapeutic agents frequently used are methotrexate and thiotepa. Cytarabine can also be used in patients with leukemias and lymphomas, but it is generally not effective against solid tumors. Radiation therapy is commonly used for localized LMD or in areas of nerve root involvement where intrathecal chemotherapy is not likely to reach adequate concentrations. Fixed neurologic deficits caused by LMD are not likely to improve with therapy, but encephalopathy may (22). The prognosis of patients with LMD is poor, with a median survival of 3 to 6 months and only a 15% to 25% chance of surviving longer than 1 year (5).
Seizures are the presenting symptom in 15% to 20% of patients with brain metastases (21). In cancer patients presenting with seizures, metabolic, infectious, and coagulopathic causes should also be considered. The initial laboratory work should include analysis of glucose level, electrolytes, blood urea nitrogen (BUN), creatinine, liver enzymes, calcium, urine analysis, prothrombin time (PT), activated partial thromboplastin time (PTT), and toxicology screening if indicated (Fig. 53-6).
Patients can have seizures during withdrawal from high-dose, short-acting benzodiazepines (such as alprazolam), alcohol, antibiotics (such as the carbapenems), pain medicines (such as meperidine), and many other medicines. The patient’s family can be helpful in sorting out the etiology of seizures by providing information about the patient’s medications, social history, and preceding symptoms, such as fever or headache. Computed tomography without and with contrast is also helpful and can identify increased intracranial pressure, bleeding, or brain metastasis. Electroencephalography (EEG) is also helpful in the evaluation of seizures and can determine whether an epileptic focus is present. Lumbar tap for CSF analysis can be helpful if the seizures are suspected to be secondary to infection or LMD, provided that there are no contraindications on brain imaging (signs of increased intracranial pressure or impending herniation) and that the convulsions have stopped.
Status epilepticus occurs when a patient has prolonged seizures with continuous seizure activity lasting >5 minutes or two or more sequential seizures without full recovery of consciousness between seizures. When patients present with status epilepticus, airway, breathing, and circulation should be assessed immediately (23,24). Initial care for patients with status epilepticus includes placing the patient in a safe environment, administering 100% oxygen by non-rebreather mask, monitoring with a continuous pulse oximeter providing suction, and administering IV fluids (normal saline). Priority should be placed on exclusion of hypoglycemia, protecting the airway, and terminating the convulsions. Anticonvulsant therapy with IV benzodiazepines (eg, diazepam, 0.2 mg/kg at 5 mg/min, up to 10 mg, or lorazepam, 0.1 mg/kg at 2 mg/min, up to 4 mg) should be administered to halt seizure activity. For patients with continuing seizures, therapy can be escalated in steps; second-line therapy includes fosphenytoin IV at15 to 20 mg of phenytoin equivalents (PE) per kilogram. Patients with refractory seizures might require high-dose anticonvulsant therapy (eg, pentobarbital, thiopental, propofol, midazolam) with complete sedation, intubation/ventilator support, EEG, and careful monitoring in the intensive care unit.
It is the general consensus of the American Academy of Neurology that routine use of prophylactic antiepileptic drugs (AEDs) for patients with brain metastases who have not experienced a seizure is not indicated (16). Once new-onset seizure in a cancer patient has been controlled, the patient should be placed on an AED. Several drugs can be used, including phenytoin, carbamazepine, clonazepam, gabapentin, lamotrigine, phenobarbital, primidone, topiramate, and valproate. Because of no significant interaction with antineoplastic drugs, some clinicians prefer levetiracetam in cancer patients.
Altered mental status is a common neurologic complaint in cancer patients, with metabolic encephalopathy being the most common cause. Altered mental status can range from a slight decrease in normal intellectual functioning to coma. A cancer patient’s mental status may change in response to several factors, such as infections/sepsis, metabolic derangements, bleeding, medications, hypoxemia, cancer therapies, paraneoplastic neurologic syndromes, and intracranial events, such as brain metastases. Organ failure, whether hepatic, renal, adrenal, thyroid, or pulmonary, can also produce fluctuations in mental status. The most common metabolic deficiencies causing such alterations are hyponatremia, hypercalcemia, hypoglycemia, and vitamin B1 deficiency. The causes of altered mental status are numerous; an extensive history and physical examination can help to identify the underlying cause and determine appropriate therapy (Fig. 53-7). The differential diagnosis and diagnostic evaluation are beyond the scope of this chapter, but a few entities are unique to cancer patients.
FIGURE 53-7
Algorithm for the evaluation and treatment of altered mental status. CT, computed tomography; ID, infectious disease; IV, intravenous; MRI, magnetic resonance imaging; RT, radiation therapy. (Adapted with permission from Yeung SJ, Escalante CP [eds]: Oncologic Emergencies. Hamilton, Ontario, Canada: BC Decker; 2002.)
For instance, cancer therapy is a common cause of altered mental status. Many neurologic manifestations, such as dementia, cognitive decline, and encephalopathy, can result from chemotherapy. Table 53-1 highlights some of the common neurologic complications of chemotherapy (5,17,25).
| Chemotherapy | Seizure | Neuropathy or Sensory Changes | Encephalopathy | Cerebellar Symptoms | Vascular Events/Stroke | Cognitive/Dementia Nerve Palsy | Cranial | Visual Changes/Loss | Myelopathy | Other |
|---|---|---|---|---|---|---|---|---|---|---|
| BCNU (carmustine) | + | + | + | + | + | + | ||||
| Busulfan | + | |||||||||
| Cisplatin | + | + | + | + | Ototoxicity | |||||
| Cytarabine | + | + | + | + | + | |||||
| Dacarbazine | + | |||||||||
| Docetaxel | + | |||||||||
| Doxorubicin | + | |||||||||
| Etoposide | + | |||||||||
| Fludarabine | + | |||||||||
| 5-Fluorouracil | + | + | ||||||||
| Gemcitabine | + | |||||||||
| Ifosfamide | + | + | + | + | ||||||
| Interferon | + | + | + | |||||||
| Interleukin-2 | + | |||||||||
| L-Asparaginase | + | + | + | |||||||
| Methotrexate | + | + | + | + | + | + | + | |||
| Paclitaxel | + | Gait abnormality | ||||||||
| Procarbazine | + | + | + | |||||||
| Tamoxifen | + | + | ||||||||
| Taxol | + | |||||||||
| Teniposide | + | |||||||||
| Thalidomide | + | |||||||||
| Thiotepa | + | |||||||||
| Vincristine | + | + | + | + | + | + | Vertigo, autonomic neuropathy | |||
| Vinorelbine | + |
Radiation therapy can also cause complications, among them leukoencephalopathy, radiation necrosis, and decreased memory and mental functioning. (The preceding section on increased intracranial pressure provides a fuller discussion of the cognitive side effects of radiation therapy.) Other possible causes of cognitive decline are narcotics (commonly prescribed for pain), infections (pneumonia, sepsis, urinary tract infection), and cerebral infarction.
Paraneoplastic syndromes are unique to cancer patients and should be considered in cases of altered mental status. In many instances, the paraneoplastic syndrome will precede the cancer diagnosis. Paraneoplastic syndromes must be differentiated from symptoms caused by progression of cancer or the side effects of cancer therapy. The two common paraneoplastic syndromes that cause mental status change through electrolyte abnormalities are hypercalcemia of malignancy and hyponatremia due to syndrome of inappropriate antidiuretic hormone (SIADH). Paraneoplastic neurologic syndromes associated with mental status changes and neurophysiologic abnormalities are paraneoplastic cerebellar degeneration and Lambert-Eaton myasthenic syndrome (5).
CARDIAC EMERGENCIES
Tumors involving the heart are much more frequently metastatic than primary. The tumors that most often metastasize to the heart are lung, breast, and GI tract cancers; leukemia; lymphoma; melanoma; and sarcoma. Metastatic involvement of the heart has also been noted in leukemia and lymphoma patients. Certain therapies can also affect the myocardium and cause pericardial disease, especially cyclophosphamide and ifosfamide at high doses, all-trans-retinoic acid (ATRA), doxorubicin, and radiation therapy (26
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree