Acknowledgments
We acknowledge the many patients, relatives, nurses, and physicians who contributed to the ascertainment of the various clinical samples reported on in this chapter.
Visit the Endocrine Hypertension: From Basic Science to Clinical Practice , First Edition companion web site at: https://www.elsevier.com/books-and-journals/book-companion/9780323961202 .


Introduction
Over the last half century, increasing rates of obesity globally have contributed toward a tsunami of more than 50 obesity-related conditions that collectively represent one of the most important threats to human health and well-being. In the United Kingdom, obesity now affects one in four adults, and overweight/obesity has become the norm, affecting the majority of the adult population, 68% of men and 60% of women [ ]. Obesity-related sequelae have a variety of complex and bidirectional etiologies that incorporate biomechanics, oncogenesis, reproductive, and psycho-social functioning. An important subgroup, with the umbrella term, “Metabolic Syndrome,” has insulin resistance as a central unifying factor that mediates the pathogenic effects of obesity (particularly visceral adiposity) commensurately on numerous facets of metabolic function. This includes primary hypertension, an important contributor to cardiovascular disease. In the United States, among the adult population, primary hypertension affects 40% with obesity, 25% with overweight, and 15% of normal-weight people [ ]. The Framingham Heart Study estimated obesity-related primary hypertension to affect 78% and 65% of men and women, respectively [ ]. Furthermore, maintaining a normal body weight appears to be an effective strategy to prevent hypertension, and current evidence suggests that weight loss reduces blood pressure in most people with hypertension [ , ].
The effects of obesity on the development of insulin resistance and primary hypertension become complicated in the context of Obstructive sleep apnea (OSA), another obesity-related condition, and the commonest form of sleep-disordered breathing [ ]. In addition to obesity contributing toward the development of OSA, there are also complex bidirectional pathogenic pathways that pertain. Severe OSA is associated with increased cardiovascular morbidity and mortality [ ].
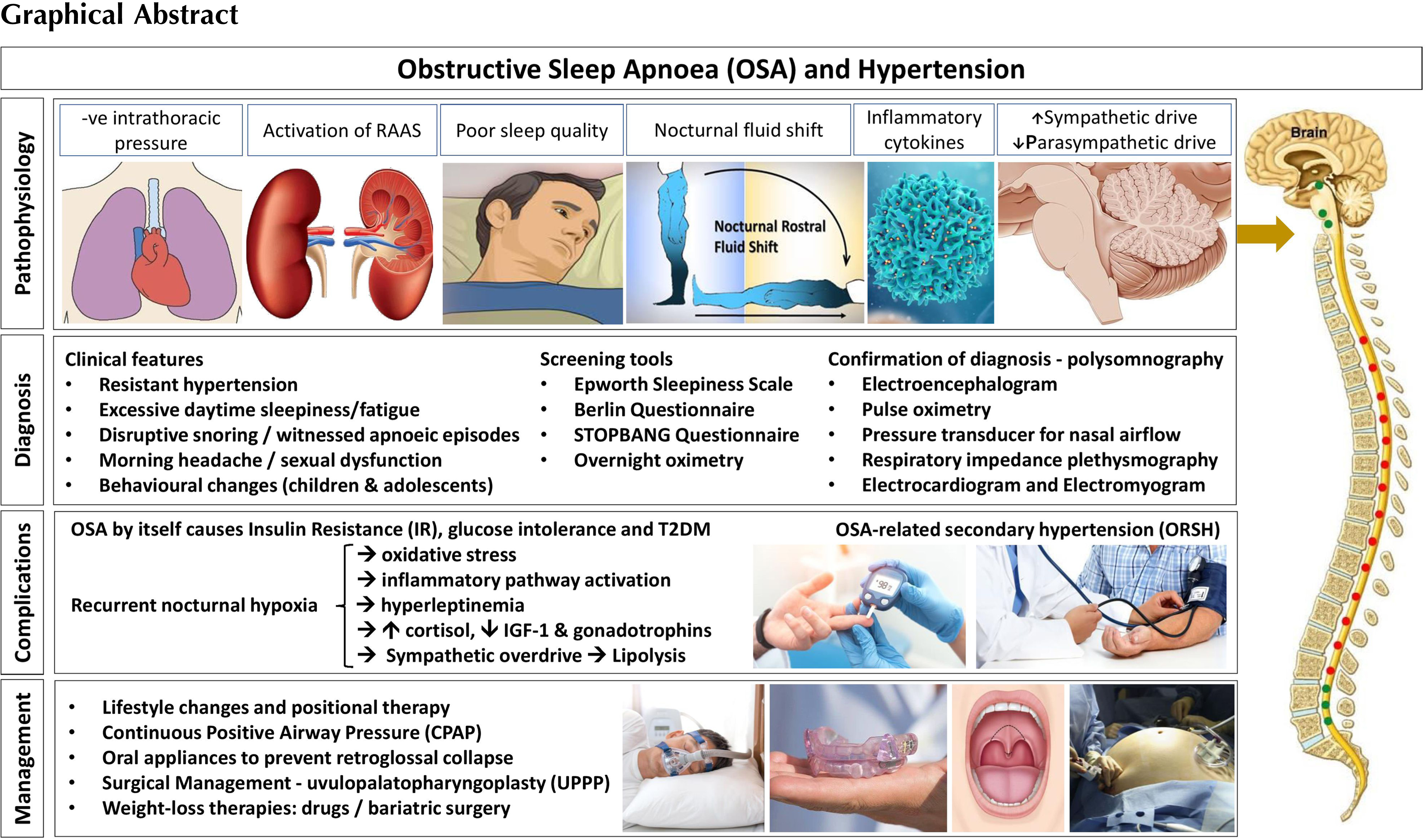
This chapter outlines the epidemiology, diagnosis, and management strategies for OSA and explores the complex interlinks that underlie obesity-related insulin resistance, OSA and ORSH.
Epidemiology of OSA
OSA is common and affects between 15% and 24% of all adults [ ]. However, the true prevalence of OSA is likely to be even greater given that OSA can remain unnoticed, relatively asymptomatic, and therefore underdiagnosed [ ]. Furthermore, the prevalence of OSA will likely increase with the growth of global populations with obesity. Rates of OSA vary according to the population studied and key demographics such as age and sex. In one systematic review of the current literature, there was an overall population prevalence of OSA (defined as at least five apneic episodes per hour) between 9% and 38%, with rates skewed toward men [ ]. Furthermore, in older populations (more than 60 years of age), the prevalence of OSA was up to 90% and 78% in men and women, respectively [ ]. For more severe OSA (defined as at least 15 apneic episodes per hour), there was an overall population prevalence of between 6% and 17%, and up to 49% in those of older age [ ]. To conclude, OSA is common among the adult population and remains underdiagnosed. Being male, older age, and the development of obesity are important risk factors.
OSA and obesity
The most important driver of OSA prevalence is global obesity [ ]. There is a close association between body weight and the development and severity of OSA, with a 10% increase in body weight elevating sixfold the risk of OSA development. Conversely, in those with OSA, its severity (measured by Apnea Hypopnea Index [AHI]) reduces commensurately with weight loss [ ]. The question then is why obesity is such an important risk factor for OSA development, and why body weight appears to predict the severity of OSA.
Essentially, weight gain and obesity (particularly in men) is associated with fat deposition in subcutaneous depots in the neck and around the upper airway [ ]. This results in narrowing and enhanced collapsibility of the upper airway (from reduced pharyngeal dilator muscle tone), with an increased propensity for apneic episodes during sleep [ ]. To compound the situation, obesity is also associated with excessive truncal fat deposition with reduced respiratory compliance (including expiratory reserve volume, forced vital capacity, forced expiratory volume, and maximum voluntary ventilation) and a corresponding reduction in alveolar–arterial oxygen gradient (even in the context of intubation and mechanical ventilation [ ]). Importantly, these obesity-associated impairments of respiratory function worsen in a person lying supine, thereby contributing toward hypoxia during sleep [ ]. Therefore, increased neck and truncal body fat in the context of sleeping in a supine position create a perfect storm in which there is increased upper airway collapsibility and impaired respiratory function. This forms the quintessence of OSA, manifesting with repetitive episodes of nocturnal apnea [ , ] driving recurrent hypoxia, hypercapnia, and surges of sympathetic overactivity associated with endothelial dysfunction, and hypercoagulability [ ]. On waking, the pharyngeal airway patency is temporarily restored until sleep resumes, and the cycle recurs numerous times each night.
The sex differences in the propensity for OSA with a ratio of about 4:1 in men versus premenopausal women [ ] merit further discussion. Crucially, on gaining weight, men tend to deposit fat in central/truncal locations (including abdomen, chest, and neck), differing from the typical deposition of fat in the thighs and buttocks in premenopausal women—the classical apple versus pear patterns of sex-related fat deposition [ ]. Furthermore, a longer airway in men, independent of body height, compared to that in women may contribute toward sex differences in upper airway collapsibility [ ]. Finally, the male pharyngeal airway, independent of Body Mass Index (BMI) and obesity, appears to be inherently more collapsible than in women due to sex-related anatomical differences, thereby further contributing toward male susceptibility to OSA [ ]. The aging process diminishes the elastic recoil, collagen content, and tethering within the upper airway [ ]. Other risk factors for OSA include ethnic origin, genetic factors, craniofacial abnormalities, and smoking (the latter due to increased inflammation of the upper airway and reduced airway sensitization affecting the arousal threshold during sleep) [ ].
Given the known changes in female body habitus, with a propensity for android fat deposition at the time of menopause, an important question relates to the risk of OSA in older postmenopausal women. Interestingly, the use of hormone replacement therapy (HRT) in postmenopausal women appears to have a protective effect with the risk for OSA similar to that in their premenopausal counterparts. Such effects of HRT may be mediated through the estrogen- and progesterone-related redistribution of fat to a more gynoid habitus [ , ]. Conversely, the absence of HRT usage in postmenopausal women appears to increase the risk of OSA, although the overall risk remains substantially lower than in age- and BMI-matched men [ ]. Regardless of menopausal status or HRT usage, obesity remains the overriding risk factor for OSA in both men and women of all ages.
To conclude this section, OSA associates strongly with obesity. Men are particularly susceptible to OSA development due to their propensity to deposit fat centrally and around the neck and to their inherent upper airway collapsibility (stemming from a longer trachea and anatomical differences compared with women). The aging process augments upper airway collapsibility. Other factors like smoking may further increase the risk of developing OSA. It should be noted that the pathogenesis of OSA is complex and remains incompletely understood. Although the biomechanical factors that underlie OSA (as outlined here) are easily understood, there are likely multiple other factors (including some that act centrally) that may contribute towards disturbed sleep in OSA and its association with obesity, which should form a focus for future research.
Diagnosis of OSA
The effective diagnosis of OSA is notoriously challenging and usually requires a proactive approach by the healthcare professional. The person suffering from OSA rarely seeks medical attention themselves, primarily because they may be unaware of their nocturnal experiences. Indeed, the main clinical features of OSA (other than resistant hypertension) include excessive daytime sleepiness and fatigue, which may have been attributed to other factors like stress. Often, it will be the partner of the person with OSA who will alert a healthcare professional to the disruptive snoring and/or witnessed apneic episodes.
The clinical assessment of OSA includes a detailed history and examination; investigations to exclude other conditions such as heart failure; accurate measurements of body weight, height (with calculated BMI), blood pressure, waist circumference, and neck circumference; and assessment for the presence of macroglossia and a crowded oropharynx. Aside from the daytime somnolence, other features of OSA may include morning headaches, sexual dysfunction, and behavioral changes (the latter particularly in children and adolescents). As a measure of daytime sleepiness, various scoring criteria are used, such as Epworth Sleepiness Scale, Berlin Questionnaire, and STOPBANG Questionnaire [ ].
The gold standard diagnostic test for OSA is polysomnography, although overnight oximetry can be used as a screener. During polysomnography, patients are monitored with an electroencephalogram (EEG), pulse oximetry, temperature, and pressure sensors for the measurement of nasal airflow (using pressure transducer), respiratory impedance plethysmography with resistance belts around the chest and abdomen to detect motion, electrocardiogram, and electromyogram sensors to detect muscle contraction in the chin, chest, and legs [ ]. Polysomnography generates substantial data. Of particular relevance to OSA are data on apnea which is defined as the absence of breathing for ≥10 s, and hypopnea which is defined as a decrease in airflow of ≥30% for ≥10 s, associated with ≥3% drop in oxygen saturations and/or detection of sleep arousal on the EEG [ ]. Such data are used to form the AHI, which determines the diagnosis and severity of OSA. The AHI simply refers to the mean average number of apneic or hypopnea episodes that occur per hour of sleep [ ], with the grading of severity shown in Table 17.1 .
| Grading of OSA severity | AHI |
|---|---|
| Mild OSA | ≥5 and <15 |
| Moderate OSA | ≥15 and <30 |
| Severe OSA | ≥30 |
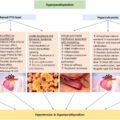
Pathophysiology of OSA-related secondary hypertension
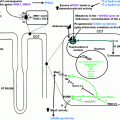
Having established the prevalence and the diagnostic criteria for OSA, its association with the obesity, and population-based risk factors, it is important to explore the pathophysiology of ORSH. Our current understanding implicates five separate processes, outlined in Fig. 17.1 . These include the following: (i) nocturnal sympathetic overdrive; (ii) chronic inflammation and oxidative stress driven by hypoxia; (iii) nocturnal fluid shifts; (iv) cardiac effects of nocturnal negative intrathoracic pressure; and (v) activation of the RAAS.
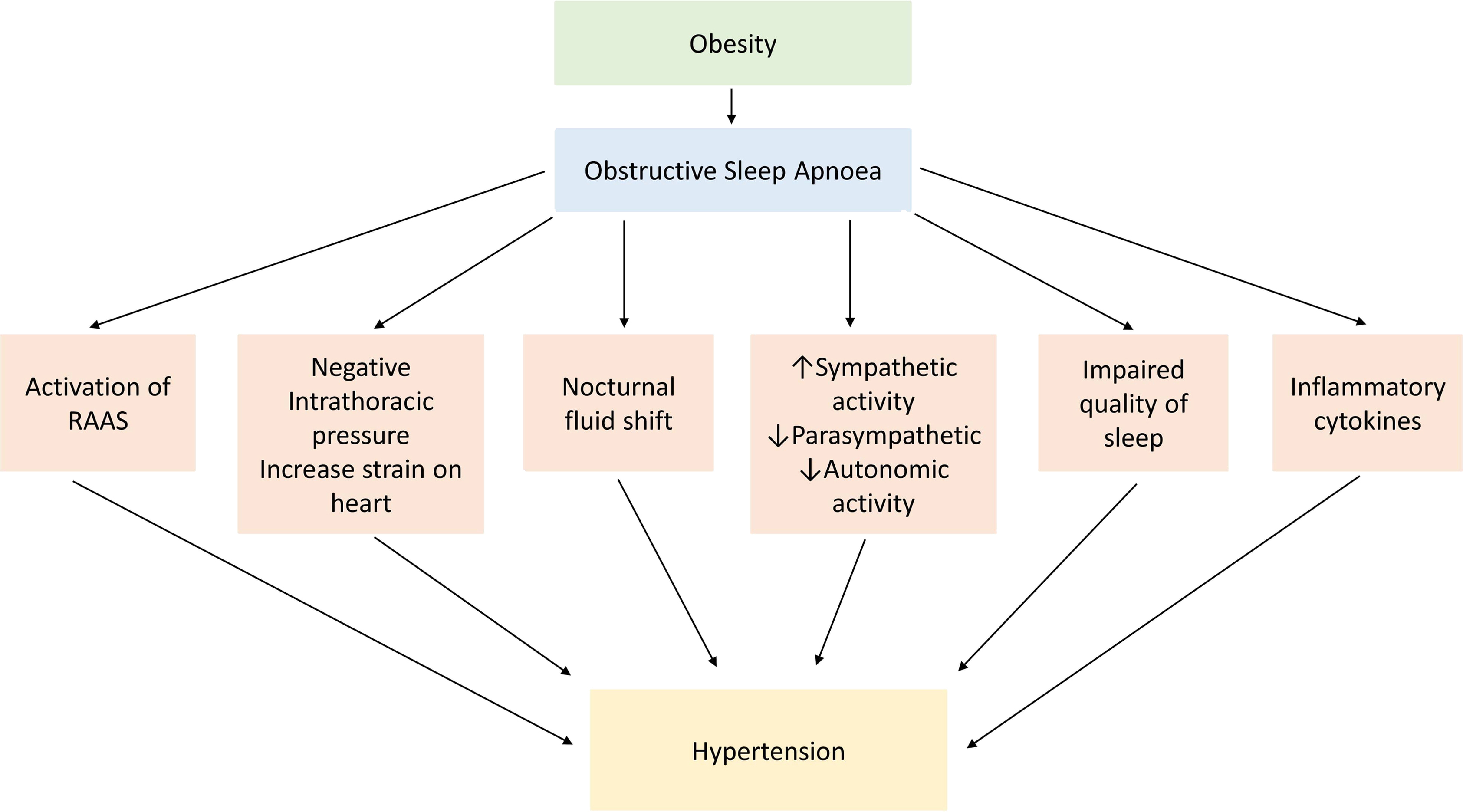
Nocturnal sympathetic overdrive
Restful sleep is usually characterized by parasympathetic overdrive, associated with a reduction in sympathetic nervous system output. This scenario occurs particularly during the nonrapid eye movement (NREM) sleep, the duration of which accounts for the greatest proportion of the sleep cycle [ ]. This sleep-induced parasympathetic dominance usually ensures that resting heart rate and blood pressure both drop during the entire duration of sleep. Interspersed between NREM sleep is the rapid eye movement (REM) sleep, with cycles of NREM and REM sleep typically occurring multiple times each night (with a cyclical duration of around 90-minutes). REM sleep is characterized by a generalized loss of skeletal muscle tone. In normal physiology and in people without OSA, such loss of muscle tone does not lead to any interference with the airway.
However, in people with OSA, this loss of muscle tone during the REM sleep contributes toward the collapse of the pharyngeal airway with resultant recurrent apneic episodes, hypoxia, hypercapnia, stimulation of carotid body chemoreceptors, and reflex sympathetic overdrive [ ]. The OSA-related sympathetic overdrive stimulates the cardiovascular system with increased heart rate and blood pressure, thereby contributing to the development and worsening of hypertension. Therefore, rather than the usual physiological drop of around 10%–15% in blood pressure that occurs during NREM sleep, in people with OSA there is typically a rise in blood pressure during sleep, with spikes in blood pressure correlating with hypoxic episodes [ ]. Importantly, in subjects with OSA, the sympathetic overdrive-mediated rise in nocturnal blood pressure that occurs following airway obstruction is sustained due to a surge in serum catecholamines [ ]. Over time, this leads to the development of ORSH [ ].
Chronic inflammation and oxidative stress driven by hypoxia
In OSA, recurrent nocturnal apnea-associated hypoxia and hypercapnia results in stimulation of inflammatory pathways and enhanced oxidative stress [ ]. This includes the release of reactive oxygen species, inflammatory cytokines, and vasoactive substances such as highly sensitive C-reactive protein (hs-CRP), interleukins (IL-1, IL-6, and IL-8), and tumor necrosis factor-α (TNF-α) [ ]. This heightened inflammatory response and the oxidative stress worsens the insulin resistance that in turn contributes toward development and worsening of hypertension and increased overall cardiovascular risk [ ]. Moreover, the inflammatory response and the oxidative stress are associated with enhanced endothelin-1 and reduced nitric oxide levels resulting in vasoconstriction and endothelial dysfunction [ ].
Nocturnal fluid shifts
People with OSA tend to have relative fluid overload compared to those without OSA, resulting from activation of the RAAS and overproduction of aldosterone [ ]. During the day, the interstitial fluid usually accumulates in the legs through gravitational effects. During the night, while supine, there is a fluid redistribution from the legs into the rest of the body, including the torso and neck. The fluid that redistributes to the neck during the night can contribute toward the narrowing of the pharyngeal airway, thereby worsening the severity of OSA [ ]. In addition, nocturnal fluid redistribution from the interstitium of the legs while supine can increase the intravascular volume load and therefore also contribute toward ORSH. These effects are particularly evident in fluid-retaining conditions like heart failure, in which nocturnal fluid redistribution can also result in venous engorgement and mucosal fluid accumulation within the neck, and further enhancing the tissue pressure around the airway [ , ].
Cardiac effects of nocturnal negative intrathoracic pressure
During apneic episodes in people with OSA, there is an increased inspiratory effort. When this occurs in the context of a blocked upper airway, there is enhanced negative intrathoracic pressure with associated increased venous return and right ventricular preload. The concurrent apnea-induced hypoxia induces pulmonary venous constriction and increased right ventricular afterload [ , ]. Apnea-induced sympathetic overdrive causes a surge in catecholamine release that has further cardiac effects through both inotropic and chronotropic mechanisms. Over time, these effects may lead to left ventricular hypertrophy, impairment in left ventricular diastolic filling, reduced stroke volume, atrial remodeling, and arrhythmias that ultimately increase the risk for the development of heart failure [ ]. The cardiac effects of apnea-induced negative intrathoracic pressure outlined here contribute toward the development of ORSH.
Activation of the RAAS
Evidence from the literature, including meta-analyses, reveals a clear association between OSA and its associated apneic episodes with the activation of RAAS, resulting in overproduction of aldosterone. In people with OSA, there are changes in the expression of the angiotensin-converting enzyme (ACE) gene, increasing susceptibility to hypertension via RAAS activation [ ]. A meta-analysis showed that people with OSA have elevated serum angiotensin II levels compared to controls, and those with ORSH have increased serum levels of aldosterone [ ]. Activation of the RAAS contributes toward the pathogenesis of ORSH through enhanced sodium and water retention, vasoconstriction, and cardiac inotropic effects [ , ]. The overproduction of aldosterone contributes to sarcopenia characterized by decreased skeletal muscle mass and function, leading to upper airway dilator dysfunction and worsening of OSA [ ].
The nocturnal hypoxia also results in accelerated loss of kidney function [ ] through hypoxia-induced tubulointerstitial injury [ , ]. Furthermore, impairment of renal function appears to increase the risk of OSA, with every 10 mL/min/1.73m 2 reduction in eGFR increasing the odds of developing OSA by around 42%, following adjustment for confounders [ ]. Primary aldosteronism also needs to be considered as OSA may coexist with primary aldosteronism in as many as 70%, and serum aldosterone levels may correlate with the severity of OSA [ ]. Therefore, it is useful to screen patients with primary aldosteronism for OSA. Although the complex bidirectional links between OSA and renal dysfunction remain incompletely understood, it is likely that the RAAS and blood pressure play important mediating roles.
OSA and insulin resistance
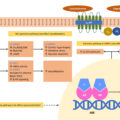
As outlined in the last section, the pathophysiology of ORSH implicates numerous mechanisms that stem from a diverse range of factors that include recurrent nocturnal hypoxia, enhanced negative intrathoracic pressure, and the supine habitus of sleeping. These factors all contribute to the development of ORSH. However, beyond blood pressure effects, OSA also contributes toward metabolic dysfunction more generally through its associated enhancement of insulin resistance as summarized in Fig. 17.2 . This includes increased risk for the development of conditions like type 2 diabetes mellitus (T2DM) [ ] and polycystic ovary syndrome (PCOS) [ ], and atherosclerotic cardiovascular sequelae like myocardial infarction and stroke.
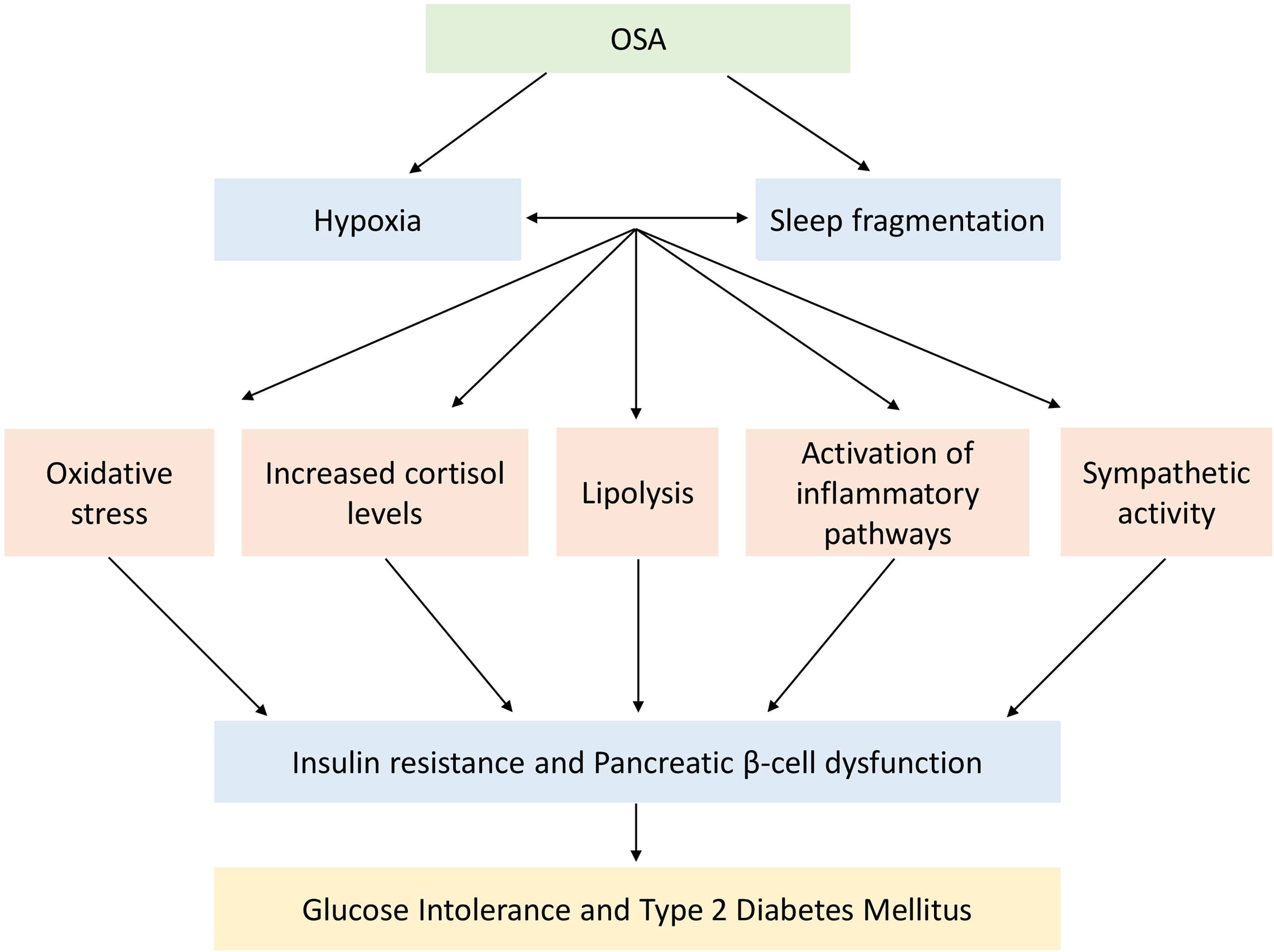
Although obesity is the main contributor to the development of insulin resistance, it is important to note that OSA worsens insulin resistance independent of any obesity-related effects. It is likely that hypoxia-induced inflammation and oxidative stress mediate this process, with observations of hypoxia-associated increase in fasting insulin levels and homeostasis model assessment of insulin resistance (HOMA-IR) [ ]. OSA-related sympathetic overdrive typically increases the levels of circulating free fatty acids and other metabolites like ceramides, diacylglycerol, and acyl-CoA via the stimulation of lipolysis [ ]. This process activates the serine kinase cascade that in turn impairs the insulin signaling and glucose transport, thereby further promoting insulin resistance [ ].
Furthermore, leptin may play complex bidirectional pathogenic roles mediating OSA and obesity with insulin resistance [ ]. It is known that hyperleptinemia and obesity-related leptin resistance are associated with insulin resistance and metabolic dysfunction [ ]. OSA appears to worsen the hyperleptinemia independent of obesity-related effects, possibly through recurrent nocturnal hypoxia [ ], with a correlation between serum levels of leptin and the severity of OSA [ ]. To further complicate the picture, the severity of OSA and associated insulin resistance can be worsened in the context of diabetic autonomic neuropathy due to the impairment of key respiratory neurons, diminished chemoreception, blunted hypercapnic, and hypoxic ventilatory responses [ , ].
Other than the effects on insulin and leptin levels and on the sympathetic overdrive, recurrent nocturnal hypoxia in OSA also impacts on other endocrine systems, which in turn may contribute toward its association with insulin resistance [ ]. Of particular relevance is the elevated serum adrenocorticotrophic hormone (ACTH) and cortisol levels in response to hypoxia-induced activation of the hypothalamic–pituitary–adrenal (HPA) axis [ , ] and its reversal in response to therapy with continuous positive airway pressure (CPAP) [ , ]. In addition to the effects of prolonged elevation of serum cortisol levels on the promotion of insulin resistance, elevated ACTH levels also stimulate the adrenal production of aldosterone that in turn contributes toward the development of ORSH [ ].
Coupled with activation of the HPA axis, hypoxia also suppresses the somatotrophin axis, with an inverse correlation between the severity of OSA and serum levels of insulin-like growth factor 1 (IGF-1) [ ]. As with hypoxia-related changes in serum cortisol, the changes in serum IGF-1 are also reversed with CPAP therapy [ , ]. Finally, OSA is associated with suppression of the gonadal axis and the development of hypogonadotropic hypogonadism [ ]. Suppressed serum levels of both IGF-1 and gonadal hormones contribute toward the development of insulin resistance.
To summarize this section, there are numerous and diverse effects of OSA on the development of insulin resistance. This includes neuronal effects from sympathetic overactivity, hormone effects of leptin and insulin, and changes in the hypothalamic–pituitary regulation of the HPA, somatotrophin, and gonadal axes. These neuro-hormonal aberrations appear to be related to hypoxia. Accordingly, correction of hypoxia with CPAP facilitates improved insulin sensitivity. This is a useful segue to switch our focus from epidemiology, diagnostics, and pathophysiology to the final section of our chapter on management strategies for OSA.
Management of OSA
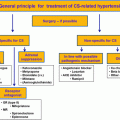
The effective management of OSA involves the establishment of improved airflow to overcome the airway narrowing and the collapsibility during sleep. Prior to this, it is important to exclude other conditions that may cause upper airway narrowing including hypothyroidism and congestive heart failure that may require separate management. Broadly, the approaches to the management of OSA include conservative management approaches; augmenting the airway patency during sleep using CPAP or oral appliances; permanent anatomical changes to the upper airway through surgery; and weight loss therapies including bariatric surgery.
Conservative management
This includes lifestyle changes to optimize the severity of OSA, such as avoidance of respiratory depressants like alcohol and opioids to limit nocturnal apneic episodes [ ]. Furthermore, avoidance of the supine position while sleeping may also help to relieve the gravitational effects on the tongue and the upper airway that is associated with increased risk of occlusion, and the increased effort of breathing while sleeping in a supine position [ ]. Such positional therapy for OSA includes encouragement of sleeping in a lateral position. High-resistance inspiratory muscle strength training, a form of physical training, has shown sustained improvements in blood pressure, arterial stiffness, endothelial dysfunction, and cardiovascular health in middle-aged and older adults with raised blood pressure [ , ].
The effective management of ORSH is important. Antihypertensive therapies may be required [ ]. Given the association of OSA with activation of the RAAS and sympathetic overactivity, ACE inhibitors, angiotensin receptor blockers, mineralocorticoid receptor blockers (spironolactone, eplerenone, finerenone), and beta blockers have a theoretical advantage over other classes of antihypertensive drugs. The mineralocorticoid receptor blockers may reduce leg to neck fluid redistribution and improve AHI; spironolactone has been shown to reduce blood pressure in patients with OSA-associated resistant hypertension reducing oxygen desaturation index, plasma aldosterone levels, AHI, hypopnea, and both clinical and ambulatory BP in one single center study [ ]. Other benefits from mineralocorticoid receptor blockers such as eplerenone include reduced neck circumference, aortic pulse wave velocity, and arterial wall stiffness [ ]. Diuretics have also been shown to have a role in the reduction of fluid displacement from the lower extremities to the neck and may have a synergistic effect with combined usage with mineralocorticoid receptor blockers [ ].
CPAP
CPAP is the gold standard management strategy for OSA [ ], which demonstrated long-term survival benefits [ ] and resolution of symptoms through diligent and continuous usage [ ], although symptoms of OSA can recur within 1–3 days of interruption of CPAP therapy [ ]. CPAP is typically used during sleep and acts as a splint; with the continuous positive pressure, it provides maintenance of airway patency and prevents the collapse of the upper airway, with immediately improved sleep and minimal side effects [ ]. The positive pressure required for upper airway patency varies between patients and according to the severity of OSA [ ]. In addition to maintaining upper airway patency, CPAP has multiple beneficial effects, including reduction of sympathetic overactivity, blood pressure [ ], free radicals, and inflammatory cytokine production [ ]. CPAP also increases serum levels of nitric oxide, a potent vasodilator essential for the regulation of vascular tone and inflammatory cascades [ , ], and downregulates the RAAS and renal hemodynamics, thereby contributing toward reduced blood pressure [ ] and glomerular pressure [ ]. CPAP therapy helps to improve insulin sensitivity and reduces vascular risk in patients with metabolic syndrome [ ], including reduced mortality from stroke by up to 8%, and from ischemic heart disease by up to 5% [ , ].
Prior to the introduction of CPAP therapy, it is important to counsel patients regarding the primary purpose of CPAP—to alleviate the symptoms of OSA. Furthermore, educational support should help to improve the adherence to CPAP, particularly in those patients with nasal difficulties. In this subgroup, strategies include the addition of nasal decongestants, heated humidification, and the use of alternative masks after exploring patient preferences (including nasal mask, a full mask, or a nasal pillow device) [ ]. Adherence to CPAP therapy is 50%–80%, with relatively few side effects, being mainly mask related (pressure-related discomfort and nasal congestion) [ ].
In addition to its longer-term usage in patients with OSA, CPAP therapy can also be used in certain acute scenarios. One example is in the perioperative management of patients with OSA, particularly in relation to the use of anesthesia and/or opioids, in which close monitoring is required due to an increased risk of reduced pharyngeal muscle tone and airway collapse. Since most surgical procedures are performed with the patient in the supine position, this may further aggravate the potential for perioperative apnea [ ]. Preoperative assessment should identify those patients who will require postoperative CPAP therapy, to improve ventilation and oxygenation and to reduce the development of respiratory complications such as atelectasis and pneumonia. CPAP therapy may also help in the management of hemodynamic fluctuations that co-occur with airway collapse [ ].
Oral appliances
Oral appliances are an alternative to CPAP therapy in people with mild and moderate OSA [ ]. A variety of oral devices exist, although the aim of each is to prevent retroglossal collapse by applying pressure to the jaw. One of the main challenges of oral devices is that several dental visits may be required over a period of time for gradual adjustment of the device, and satisfactory outcomes may take up to 9 months to occur [ ]. However, when used successfully, oral devices confer beneficial effects on reducing the systolic and diastolic blood pressure, and mean arterial pressure [ ].
Surgical management
Surgical management of OSA is usually considered in those who do not adhere to, comply with, or tolerate CPAP therapy [ ]. The main surgical approach is uvulopalatopharyngoplasty (UPPP) which is often effective at reducing the AHI [ , ]. Tonsillectomy with UPPP has also been shown to improve symptoms of OSA [ ]. It should be noted that any surgical approach to the management of OSA may also require the reintroduction of CPAP therapy as surgery may improve, but not actually eliminate the upper airway collapse due to nonanatomical factors such as pharyngeal muscle tonicity [ , ]. Although the reintroduction of CPAP may seem problematic postsurgery, procedures like modified UPPP reduce retropalatal obstruction, preserving the palate and velopharyngeal sphincter functions, thereby minimizing any potential complications of CPAP therapy [ ]. Other surgical approaches to the management of OSA include maxillomandibular advancement where both upper and lower jaws are advanced surgically to increase the space in the oropharynx, though this procedure may be less successful in patients with larger necks [ ]. In extreme cases of OSA, treatment with a tracheostomy may be required to bypass the oropharyngeal obstruction [ ].
Weight loss therapies
The most effective management strategy for OSA is through effective and sustained weight loss. It is beyond the scope of this chapter to provide a detailed exposition of the management of obesity. In summary, this consists of lifestyle therapies including dietary modification combined with physical activity; pharmacotherapies; and bariatric surgery; often coordinated through a multidisciplinary approach. In addition to a reduction in fat tissue around the neck and therefore improved upper airway patency, effective weight loss will also reduce adiposity around the trunk and chest, therefore reducing the effort of breathing, particularly when supine [ ].
Perhaps the most effective long-term strategy for the management of OSA is through bariatric surgery. In one meta-analysis, it was shown that bariatric surgery results in the resolution of OSA in around 85% of patients overall [ ]. Of course, morbid obesity with OSA often occurs in the context of multiple other metabolic dysfunctions [ ]. In this common scenario, bariatric surgery, through achieving substantial and sustained weight loss, often represents an excellent treatment option for metabolic dysfunction. In addition to the metabolic benefits of weight loss, resolution of OSA will also independently benefit metabolic status due to its independent effects on insulin resistance as outlined earlier. Finally, effective reduction of adiposity from the abdominal region following bariatric surgery reduces pressure on the inferior vena cava, thereby improving the venous return and reducing activation of the RAAS, thereby improving blood pressure and microalbuminuria.
Conclusions
Within the realm of obesity-related conditions, OSA occupies an important position. Its development often goes unnoticed, with nonspecific features of tiredness and fatiguability which may be attributed to other factors such as stress. This highlights the importance of the healthcare professional taking a proactive role in the screening and diagnosis of OSA, and the importance of gaining insight from sleeping partners, regarding witnessed snoring and apneic episodes. In short, as healthcare professionals we need to heighten our suspicions when it comes to OSA given its elusiveness and underdiagnosis, its association with dysmetabolic and cardio-respiratory dysfunction, and the potential benefits from effective treatment. Screening for OSA through questionnaires like Epworth Sleepiness Scale takes very little effort and time and can be repeated easily at annual clinical assessments and well-being checks, especially in those patients at increased risk, including obesity and other dysmetabolic conditions. Those identified to be at high risk of OSA from screening need referral for polysomnography.
OSA contributes toward metabolic dysfunction, including ORSH and insulin resistance through obesity-independent effects, likely implicating hypoxia. This is important given that those with obesity who also develop OSA have a metabolic double-whammy that requires focused effort from healthcare professionals regarding close monitoring for the development of metabolic dysfunction (such as regular measurements of blood pressure, HbA1C, and serum lipid profile), and the early institution of effective management strategies. As outlined, there are numerous factors that mediate links between obesity and OSA with ORSH and insulin resistance that include diverse neuro-endocrine pathways.
Aside from the importance of proactivity in our approach to the screening and diagnosis of OSA, a key learning point from this chapter is that OSA is eminently treatable. Given its association with obesity, it is no surprise to learn that effective and sustained weight loss, especially following bariatric surgery, represents the best management strategy for OSA. However, numerous other treatment strategies are available, including the use of CPAP therapy. Our aims when managing OSA are to improve overall well-being including reduced tiredness and fatiguability, but also to improve future cardiovascular risk, and to optimize cardiac, respiratory, renal, and vascular functionality. A management strategy that includes a focus on successful and sustained weight loss will provide further health benefits that extend well beyond merely treating OSA per se.
Finally, when managing OSA, we should always be mindful that behind every diagnosis there is a person, with the possibility of a partner, a family, job, friends, and inner and outer social groups. The implications of OSA on that person and the people and institutions associated with them should be considered carefully and empathically on an individual basis. Their management should be tailored accordingly. Although the effective management of OSA can be challenging, it is also one of the most satisfying conditions to treat across the whole realm of medicine, with the possibility of literally transforming someone’s life (and that of partners and families, etc.). This message of hope should act as our guiding light to motivate us to do what is right for our patients in the timely and effective diagnosis and management of OSA.
Learning points
- •
OSA is a common obesity-related condition that is likely underdiagnosed.
- •
OSA associates independently with worsening insulin resistance and is a risk factor for metabolic dysfunction including hypertension through multiple pathophysiological mechanisms implicating nocturnal sympathetic overdrive, inflammation-mediated effects of hypoxia, nocturnal fluid shifts, cardiac effects, and activation of the RAAS.
- •
Given its strong association with metabolic dysfunction and its eminent treatability, prompt diagnosis of OSA is important through simple questionnaire screening tests and polysomnography.
- •
Among the multiple management strategies for OSA incorporating conservative and surgical approaches and CPAP, effective and sustained weight loss remains key.
References
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree