Fig. 13.1
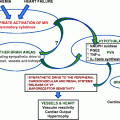
Putative mechanisms for development of obesity-hypertension (OH)
Table 13.1
Mechanical and humoral factors implicated in OH
Hyperinsulinemia and insulin resistance |
Chronic noninfective inflammation |
Chronic adipocytokinemia (elevated local adipose and systemic interleukin 6 [IL-6], interleukin 1 [IL-1], tumor necrosis factor alpha [TNF-α], and plasminogen activator inhibitor 1 [PAI-1] levels) |
RAAS activation (local adipose, renal, and systemic) |
Sympathetic nervous system activation (local adipose, renal, and systemic) |
Reduced atrial natriuretic peptide levels |
Endothelial dysfunction and oxidative stress |
Elevated endothelin levels |
Elevated resistin levels |
Hyperuricemia |
Hypoadiponectinemia |
Leptin resistance and hyperleptinemia |
Elevated free fatty acids levels and hypertriglyceridemia |
Overexpression of 11 beta hydroxysteroid dehydrogenase type 1 enzyme activity |
Glomerular hyperfiltration and intraglomerular hypertension |
Microalbuminuria |
Increased renal capsular and intra-abdominal pressure |
Renal sodium retention and impaired pressure natriuresis |
Obesity-associated glomerulopathy (focal segmental glomerulosclerosis) |
Obstructive sleep apnea |
Increased renal sodium retention and expanded blood volume are central to OH. Among the means by which this is presumed to develop are increased sympathetic nervous system (SNS) activity as well as increased RAAS activity presumably augmented by the excessive production of angiotensinogen from the increased adipocyte cell mass [47, 48]. It also appears that increased intrarenal pressure associated with abdominal obesity contributes to this process [47–49]. The increased renal sodium retention impairs renal pressure natriuresis which in and of itself elevates systemic blood pressure but additionally obesity is associated with increased renal plasma flow, increased glomerular filtration rate, increased glomerular pressure, and increased renal tubular reabsorption which are the result at least in part of the hyperactivated RAAS and SNSs [48, 50–52]. These derangements all reduce overall glomerular reserve and encourage the early development of glomerular-based proteinuria even in the absence of the focal segmental glomerulosclerosis known to be associated with marked obesity [48, 50–52].
Sympathetic Nervous System Overactivity
SNS overactivity appears to play a central role in the development of OH. Cross-sectional data show that BMI and plasma norepinephrine levels are independent predictors of blood pressure levels especially among obese hypertensives [48, 50–52]. Obese subjects compared with age matched lean individuals have been shown to have elevated plasma norepinephrine levels and muscle sympathetic nerve activity [48, 50–52]. Beyond the systemic increased SNS activity, however, it has also been demonstrated that obesity is associated with local regional SNS overactivation in the kidneys and adipose tissue [53]. Furthermore, it has also been shown clinically that combined alpha and beta blockade results in better blood pressure control among obese than lean hypertensives [54]. It also appears that the SNS overactivation may play a role in the hyperleptinemia and hyperinsulinemia associated with obesity since the degrees of these improve with pharmacologic alpha and beta blockade [55]. Other potential mediators for the observed SNS overactivation include angiotensin 2, aldosterone, FFAs, and adiponectin.
Leptin
Leptin which is the product of the OB gene is predominantly expressed and produced by adipocytes in proportion to the total fat mass [45, 56–58]. While its established actions are mainly at the hypothalamus where it induces satiety and increased energy expenditure, it is known to have several other less well-characterized actions on various tissues including enablement of menarche, anabolic action on bone, and sympathetic activation of several tissues [45, 56–58]. This SNS hyperactivation is capable of inducing hypertension as has been demonstrated in rodent models where chronic leptin infusions cause sustained hypertension which can be prevented by combined beta and alpha blockade [59]. Several experimental models of obesity including the agouti obese and diet-induced obese mice both show selective leptin resistance involving its effects on metabolic rates and satiety in the hypothalamus but preservation of its SNS activatory effects and this appears to also often occur in typical human obesity [60–63].
Renin–Angiotensin-Aldosterone System
The renin–angiotensin-aldosterone system (RAAS) has been implicated in the etiopathogenesis of OH-based multiple clinical observations and the results of intervention trials using RAAS inhibitors and modulators in subjects with OH. Obese subjects are known to have elevated serum angiotensinogen, rennin, and angiotensin converting enzyme activity levels [64]. It has also been demonstrated that adipose tissue is capable of both paracrine and endocrine angiotensinogen production, thus providing one plausible mechanism for the increased angiotensinogen levels observed in obesity [65–68]. Angiotensin 2 which is the most vasoactive product of angiotensinogen exerts autocrine, paracrine, and endocrine effects that include increasing sodium retention, and inhibiting pressure natriuresis in addition to consequent volume expansion and blood pressure elevation [65–70]. It has also been shown that use of angiotensin concerting enzyme inhibitors (ACE-I) reduce not only sodium retention but also blood pressure in animal models of OH and in obese hypertensive human patients [71, 72]. Furthermore it has also been demonstrated that weight loss in obese subjects is associated with reduction in both the adipose tissue expression of angiotensinogen and circulating levels of angiotensinogen, renin, ACE-I, and aldosterone levels which is also accompanied with reduction in blood pressure levels [73, 74]. Aldosterone which is also known to be elevated in obesity and especially visceral obesity plays a critical role in the RAAS overactivity associated with obesity and in the development of OH [65, 73, 75, 76]. The basis of the hyperaldosteronism observed in OH is still the subject of ongoing investigation and speculation. The direct effect of adipocyte-derived angiotensinogen on systemic aldosterone production as well as the role of recently identified adipocyte-derived mineralocorticoid factors on adrenal aldosterone production appear to be among the underlying mechanisms involved [77]. There is also an emerging association now recognized between obstructive sleep apnea (OSA) which is quite prevalent in obesity and a clinical state of primary hyperaldosteronism [75, 76, 78]. The specific aldosterone receptor antagonist Eplerenone has been demonstrated to attenuate or prevent the onset of hypertension in obese dogs on a high fat diet [79] and adds to the available data that indicates the important role of aldosterone in OH. Similar findings in human cohorts confirm that this is also relevant to the pathophysiology of OH in humans [80–82].
Cortisol
The role of cortisol in the pathogenesis of OH is still unclear. Cushing’s syndrome, a state of sustained systemic hypercortisolemia, is clearly associated with hypertension, visceral obesity, profound insulin resistance, and other features of the metabolic syndrome but sustained autonomous systemic hypercortisolemia has not been demonstrated in non-syndromic obesity [83]. There is, however, data that suggest overactivity of 11 beta hydroxysteroid dehydrogenase type 1 (11BHSD-1) in omental adipose tissue of obese subjects [84–86] which by increasing local tissue cortisol production may result in a “visceral Cushing’s syndrome” like state sometimes referred to as “Omental Cushing’s” [87]. The arena of tissue-specific inhibitors of 11BHSD-1 is still in phase 1 and 2 development but could ultimately prove to be another management option for management of resistant OH.
Hyperinsulinemia and Insulin Resistance
Hyperinsulinemia which is generally presumed to be compensatory for underlying tissue insulin resistance is highly prevalent though not universal to obesity. It has been suggested as another pathogenetic player in the development of OH [88, 89]. It is, however, difficult to tease apart the independent role of hyperinsulinemia in OH that is not interlinked to the often accompanying hyperleptinemia, RAAS activation, and SNS overactivation. In animal models, insulin is known to cause SNS activation in the absence of symptomatic hypoglycemia, RAAS activation, and increased renal sodium retention [25, 47]. The available human epidemiologic data, however, shows a less robust association between hyperinsulinemia and hypertension [90]. There is also human data that demonstrates that while hyperinsulinemia increases SNS activity, it is also associated with vasodilation and no accompanying blood pressure elevation [91]. One disease model that provides the opportunity to evaluate the potential role of hyperinsulinemia on its own without accompanying underlying insulin resistance and the metabolic syndrome is the clinic state of patients with insulinomas who have sustained fasting and postprandial hyperinsulinemia. Investigation of these patients has, however, not shown consistent SNS hyperactivation nor hypertension [92] suggesting that underlying insulin resistance rather than absolute hyperinsulinemia may be the critical basis for the observed association between hyperinsulinemia and OH. The elevated FFA levels that often accompany insulin resistance states are known to be associated with SNS hyperactivation and hyperglycemia often associated with insulin resistance can also deleteriously affect vascular compliance and increase the risk for development of hypertension [93, 94].
Adiponectin
Adiponectin is distinctive among adipokines in that in contrast to most others its adipose tissue expression is downregulated and its serum circulating levels reduced in the setting of excess adiposity associated with obesity [95]. Adiponectin has been suggested to be an independent risk factor for hypertension in both lean and obese subjects [95, 96]. It appears that hypoadiponectinemia induces activation of the adipose tissue RAAS and that SNS overactivity in turn reduces adiponectin gene expression providing links between hypoadiponectinemia and both RAAS and SNS overactivity [95, 96]. These may be the underlying basis for hypoadiponectinemia-associated hypertension.
Endothelin
Endothelial dysfunction is common in both obesity and hypertension [95–97]. It is also almost universally associated with systemic insulin resistance. The endothelial vascular tone is largely regulated by the balance between vasodilatory and vasoconstrictive endothelial vasoactive humoral agents of which nitric oxide (NO) and endothelin 1 (ET-1) are the major contributors [95–98]. ET-1 activity is known to be increased in subjects with OH and an association has been described between blood pressure levels and gene polymorphisms of ET-1 among obese Japanese subjects [99–102]. It appears that endothelin’s role in the development of OH is the result of both the direct vasoconstrictive effects of the peptide and the reduced NO levels that occur in OH [98, 103]
Renal Capsular Pressure
The increased intra-abdominal and peritoneal fat has been demonstrated to mechanically induce increased intra-abdominal pressure [104]. Similarly, the increased perirenal fat has been implicated in inducing increased intra renal pressure [7]. This increased pressure has been demonstrated to be associated with impairment of renal-based pressure natriuresis and increased renal interstitial pressure as well as fluid retention all of which cause blood pressure elevation [105, 106]. The raised intrarenal pressure may also be part of the basis for the known better correlation between abdominal obesity and OH compared to lower body (so-called gynecoid) obesity (which is dominantly in the hips and thighs) and OH [49].
Other Renal Structural and Functional Changes
Beyond the known effects on the kidney from increased intrarenal pressure highlighted above, obesity induces a host of other structural changes which all conspire to result in blood pressure elevation. It has been shown amongst others that obesity causes marked afferent renal artery vasodilation and increased glomerular filtration as well as intraglomerular pressure [107]. It also appears however that the chronic renal vasodilation associated with obesity causes chronic elevated hydrostatic glomerular pressure which along with the hyperlipidemia and hyperglycemia often accompanying obesity induces glomerulosclerosis and progressive nephron loss which in time can lead to chronic renal disease and renal failure [49, 69, 107]. Focal segmental glomerulosclerosis is distinctively associated with obesity and is distinct from the idiopathic focal glomerulosclerosis seen in the general population [108, 109]. This distinct entity which is associated with a progression to end stage renal disease in the absence of intervention is also called the obesity-related glomerulopathy and is associated with coexisting glomerulomegaly and the development of hypertension [109]. Unlike the idiopathic form of focal segmental glomerulosclerosis it is far less commonly associated with the nephrotic syndrome and more indolent in clinical course. Clearly obesity is associated with development of renal damage, hypertension, and progressive nephron loss but this quickly initiates a positive reinforcing cycle whereby the progressive renal damage further worsens the blood pressure which accelerates the renal damage ultimately leading to end stage renal disease in the absence of clinical intervention.
Free Fatty Acids
Elevations in both fasting and postprandial FFA levels are well described in association with obesity and insulin resistance states [40, 110, 111]. It is believed that FFAs raise blood pressure by increasing SNS activity and also possibly by SNS vascular responses [44, 112]. It has been demonstrated that acute elevations in FFA following intravenous lipid infusions increase vascular reactivity to α agonists. Infusions of oleic acid for example either systemically or into the portal circulation in rat models increase both heart rate and blood pressure and these effects are ameliorated by adrenergic blockade [113, 114]. Portal vein infusions generally cause greater blood pressure elevations than systemic infusions suggesting an additional role for afferent pathways in the liver for mediating the hypertensive effect of FFAs. Similar findings though not as dramatic in effect size have been documented in humans following infusions of intralipid along with heparin [44, 115]. The long-term effects of chronic serum FFA elevation are still unclear and largely unstudied in humans [44, 115].
Resistin
Resistin is a relatively new identified adipocyte-derived peptide that has an insulin antagonistic effect in vivo and in vitro [116]. Serum levels of resistin are elevated in both dietary induced and genetic models of obesity. The exact role of resistin in OH is still controversial with reports on the level and degree of association yielding conflicting results [117, 118]. It does appear, however, that at least among the Chinese, certain resistin gene polymorphisms may be associated with a significantly increased blood pressure burden [117].
The Atrial Natriuretic Peptide System
The system of natriuretic peptides include the atrial natriuretic peptide (ANP) and the brain and C type natriuretic peptide. They are produced predominantly in the heart musculature, brain, blood vessels, and the kidney and are each encoded by separate genes and act on specific receptors [119–122]. The overall effects of these peptides include induction of natriuresis and diuresis with consequent reduction in plasma volume and renal sodium retention in clinical states associated with extracellular volume expansion. They also reduce sympathetic tone and blood pressure as part of their activity profile. Not unexpectedly based on this activity profile it is known that transgenic mice that overexpress the ANP gene have lower blood pressures while those with ANP gene knockout develop salt-sensitive hypertension [123–125]. It has been shown that compared to obese normotensive subjects, subjects with OH have lower ANP plasma levels. These patients also demonstrate a greater blood pressure reduction response to exogenously administered ANP [126]. These salutary effects are also even more pronounced in the setting of accompanying low calorie diet intake [127] In addition, it has been observed that the typical ANP surge associated with salt loading in lean subjects is markedly blunted. Plasma renin activity and aldosterone levels are typically suppressed in obese subjects following saline infusion, and these observations provide some mechanistic explanation for subjects with OH being particularly more salt sensitive than lean or non-hypertensive obese subjects [128]. The magnitude of the role ANP plays in the onset and development of OH is still the subject of ongoing study; however it is clear that impaired ANP action, overexpression of the natriuretic peptide C receptor (which increases ANP clearance), reduced ANP levels, and certain polymorphisms of the natriuretic peptide C receptor gene are all associated with hypertension [126, 127, 129].
The Role of Genetics
Genetics likely explains the clinical observation that while OH is common it is not universal to subjects with obesity. Ethnicity as a poor surrogate for genetic endowment shows some disparity in the degree and prevalence of OH even with comparable weight gain [44, 112, 130–132]. The Pima Indians are an example of a population with profound propensity for obesity and diabetes in whom the prevalence of hypertension is not correspondingly high unless they develop advanced diabetic nephropathy. African American women (probably consequent on their differences in adipose distribution) are also observed to show a lower prevalence and degree of hypertension than Caucasian women with similar degrees of weight gain [130–133].
Cardiovascular Consequences of Obesity-Hypertension
Sustained blood pressure elevation combined with coexisting obesity over a sustained period of time eventually results in deleterious hemodynamic and structural changes. Among the most prevalent structural change associated with OH is concentric or eccentric left ventricular hypertrophy (LVH) [23, 89, 111, 134, 135]. When pressure overload dominates the result is concentric LVH while eccentric LVH is the dominant pattern when volume overload is the major underlying hemodynamic feature. It appears that this LVH increases the risk for heart failure and dysrhythmias [23, 89, 111, 134, 135]. The dysrhythmias in particular may be the basis for the known increased risk for sudden cardiac death among persons with OH [136].
Because of the common coexistence of the distinctive dyslipidemia of the metabolic syndrome and insulin resistance in the setting of OH it is also not surprising that the risk for ASVD and in particular myocardial infarctions appears increased in OH compared to subjects without hypertension as is eloquently demonstrated by data from the PROCAM study [112].
Beyond the effects of OH on the heart, its impact on the kidneys are also well documented. The association between obesity and progressive nephron loss as well as focal segmental glomerulosclerosis has already been previously highlighted. Data from the Framingham cohort suggest that for every unit increase in BMI after a mean follow-up of ∼18.5 years, there is an accompanying 1.2× increase in the risk for accompanying renal disease [137–139].
OH is associated with a proinflammatory cytokine milieu which is largely driven by the host of inflammatory adipokines known to be overexpressed in the increased tissue fat mass associated with OH [112, 133]. Among the central players in this regard are TNF-α, C-reactive protein, IL-1 and 6, and the acute phase reactants fibrinogen and PAI-1 [112, 133, 140–142]. While the association between obesity and a chronic inflammatory state is well established, a direct cause and effect relationship has not been established and the relationship of these cytokines and adipokines to the development and onset of OH is unclear. It is also unclear if treatment with nonsteroidal anti-inflammatory or other anti-inflammatory agents would reduce the levels of these cytokines and adipokines and whether this would ultimately impact ASVD risk in obese subjects. What is apparent, however, is that this inflammatory milieu adds to the ASVD risk associated with obesity and OH. There is available data that demonstrates the ability of the anti-inflammatory agent salsalate in improving indices of insulin resistance and glycemic measures in diabetes [143–147].
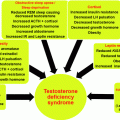
Obesity and OH are also associated with hypercoagulability and increased risk for thromboembolism. This is multifactorial in origin including the association of obesity with polycythemia and the prothrombotic milieu resulting from the increased levels of fibrinogen, factor 7 antigen, protein C, and PAI-1 [148, 149]. Leptin appears to play an enabling role in the development of this prothrombotic mix. The complex interrelationships and consequences of OH on cardiovascular risk are further detailed in Fig. 13.2.
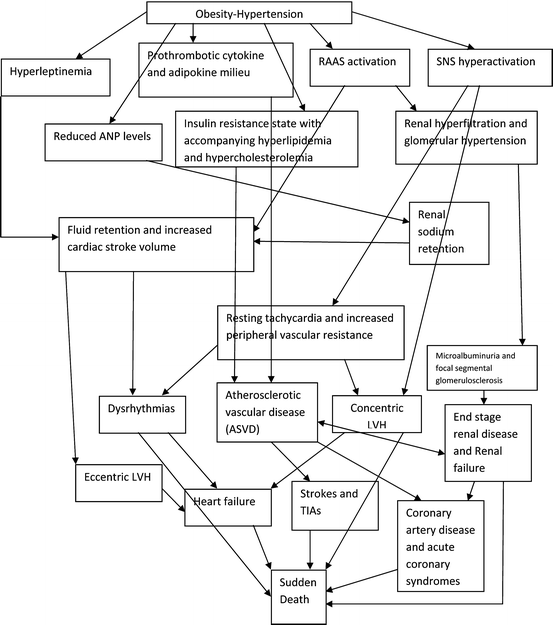

Fig. 13.2
Cardio-renal and cerebrovascular consequences of OH
Differential Diagnostic Considerations
OH is quite prevalent, however, when a hypertensive subject under clinical evaluation is also found to be obese it is important for the clinician not to assume that this is simply OH with no other complicating issues involved. This is especially true if the patient’s clinical presentation is with either resistant hypertension or a hypertensive urgency/emergency. Given the prevalence of obesity in the general population it is important to be aware that virtually all the identified causes of secondary hypertension including rarities like pheochromocytoma can and have been described in obese subjects and can thus be mistaken for regular OH if careful clinical inquiry by detailed history taking and comprehensive physical is not done. Among the important endocrine considerations to be aware of that can present akin to typical OH are (a) clinical hypothyroidism which though not typically associated with marked obesity is often associated with a volume expanded hypertensive state, (b) Cushing’s syndrome in which the degree of abdominal/visceral obesity and the relative atrophy of the extremities as well as the distinctive striae (violaceous, raised, and wide as opposed to the pale, thin striae albicante from mere abdominal distension), (c) acromegaly where the distinctive facial bony overgrowth, macroglossia, and spade like hands and feet may be the clinical findings which raise this possibility, (d) Hypopituitarism or hypothalamic obesity where the clinical history is critical in making the correct diagnosis as well as (e) insulinoma where the distinctive history of spells suggestive of recurrent hypoglycemia is central to the clinical suspicion.
While still poorly characterized, the entity of subclinical Cushing’s syndrome is another diagnostic consideration that clinicians need to be aware of when evaluating the subject with obesity and hypertension [150–153]. These patients often clinically resemble subjects with typical obesity with features of the metabolic syndrome; however, careful testing of the hypothalamic–pituitary-adrenal axis demonstrates the presence of significant autonomous hypercortisolemia which has clinical consequences demonstrated the prevalence of dyslipidemia, insulin resistance, ASVD, osteopenia/osteoporosis, and hypertension in these subjects [154, 155]. These patients also have a prevalence of so-called “adrenal incidentalomas” exceeding that of the general population [152–155]. There is some controversy as to whether the adrenal nodules found in this setting are truly incidentalomas by strict definition as there are studies that show the resolution/improvement of the hypercortisolemia and many of its clinical sequelae including hypertension following adrenalectomy that removes the nodule(s) in question [152–155].
Obstructive Sleep Apnea and Obesity-Hypertension
Obesity and in particular upper body obesity is known to be a major risk factor for OSA [156]. Beyond this, however, OSA also often coexists with hypertension; while it has been estimated that ∼5–10% of the general population have OSA it is estimated that ∼50–60% of hypertensive subjects have OSA [156]. Furthermore, there is extensive evidence indicating that OSA is highly prevalent among subjects with obesity and the metabolic syndrome [157–160]. The association between hypertension and OSA is suggested to be possibly causative based on prospective observation of subjects in the Wisconsin Sleep cohort study which demonstrated that after adjustment for all major possible covariates, a prospective dose–response association exists between the degree of sleep disordered breathing at baseline and the degree of hypertension at follow-up ∼4 years later [161–164]. There is also a considerable body of data that suggests that OSA is markedly under recognized and also often under- or untreated even when identified [161–164]. The clinical spectrum of presentation of OSA is quite broad and includes snoring, daytime somnolence, early morning headaches, fatigue, and frequent sleep time arousals with hypopnea and/or apneic spells [76, 78, 158]. On sleep laboratory testing findings may include demonstrable sleep time hypoxia, hypercapnia, restless leg syndrome, and frequent arousals. The frequent arousals are often associated with systolic blood pressure spikes and a loss of the normal physiologic blood pressure dip associated with sleep [76, 78, 158]. While polysomnography remains the gold standard method for confirmation of the diagnosis of OSA, there are several available and well-validated screening methods for assisting clinicians in identifying patients who should have more in-depth evaluation. Measurement of neck circumferences, inspection of the airways, and grading of the oropharynx using scales such as the Mallampati scale as well as use of validated questionnaires such as the Epworth sleepiness scale are the most popular clinically accessible instruments [165, 166].
The exact means by which OSA induces hypertension is still the subject of ongoing clinical investigation. It does appear that the association between OSA and hypertension is beyond mere statistical association and seems to be such that OSA is associated not only with development of but also worsening of preexisting hypertension [76, 78, 158]. Among the suggested means by which OSA induces hypertension is its known association with SNS overactivity [167–170]. Animal models of OSA have also demonstrated that intermittent hypoxia is associated with sustained hypertension which can be ameliorated by surgical denervation of peripheral chemoreceptors, adrenal demedullation, and/or chemical denervation of the peripheral SNS [171–173]. It has also been shown that continuous positive airway pressure therapy (CPAP) using face masks which is the most reliably consistent treatment strategy for OSA is associated with reduction in SNS activity as well as improvement in resting tachycardia with consequent improvement in the degree of hypertension [156, 174].
Beyond the impact of SNS overactivity, however, it is also apparent that OSA is associated with endothelial dysfunction though it is still unclear whether this is an initiating factor or consequence of OSA [175]. Endothelium-dependent vasodilation is impaired in patients with OSA and improves with CPAP treatment [176, 177]. Endothelin-1 (ET-1) which is endothelium derived appears to play a causative role in the induction of hypertension in OSA [178]. It has been demonstrated that patients with OSA have higher plasma ET-1 levels than health controls and in addition the nocturnal ET-1 levels correlate significantly with the severity of the OSA [178]. Furthermore, CPAP treatment of OSA subjects has been demonstrated to reduce ET-1 levels and the degree of ET-1 level reduction also correlates significantly with the degree of salutary changes in mean arterial blood pressure and oxygen saturation [179].
Recent data also suggests that aldosterone and angiotensin 2 also play a prominent role in the development of hypertension in OSA. Plasma angiotensin 2 and aldosterone levels are elevated in OSA patients compared to controls. CPAP therapy has also been shown to be associated with correlated improvements in blood pressure, plasma renin activity, and plasma angiotensin levels [180]. The activation of the RAAS system in subjects with OSA has features consistent with primary hyperaldosteronism and the triad of hypertension, primary hyperaldosteronism, and OSA is now well recognized [181–184]. It has also been demonstrated that treatment with aldosterone antagonists ameliorates not only the hypertension associated with OSA but also indices of OSA severity as well [181–184]. Animal models of episodic hypoxia have also demonstrated that the hypertension associated with this is prevented when the renal arteries of such animals are denervated and angiotensin receptor blockade done chemically [185].
Management Strategies for Obesity-Hypertension
Lifestyle modification strategies are particularly important and central to the effective sustained management of OH. The trial of antihypertensive intervention and management (TAIM) demonstrated that weight loss of greater than 4.5 kg or ∼5% of baseline weight in the studied cohort achieved blood pressure reductions comparable to that from a single optimally dosed antihypertensive agent [190]. There are other studies that have replicated these findings [43].
The Place of Weight Loss in OH Management
Sustained weight loss achieved has a salutary effect on blood pressure in OH irrespective of the means by which this is achieved. The central principle for dietary strategies for weight loss is the reduction of overall caloric content while increasing overall fiber content [191–195]. While there are many different strategies by which this may be achieved, reducing portion sizes, proper eating hygiene (regular meals and timing with minimized caloric snacking), and reduced fat and simple carbohydrate content in meals are the most robust strategies for achieving sustained weight loss [196, 197]. The Framingham study has demonstrated as also shown in the TAIM study that weight loss targets required to significantly impact blood pressure specifically and cardiovascular risk surrogates generally are relatively modest. Weight loss of 5 lbs or more can reduce ASVD risk estimates by ∼40% [198]. The benefits of weight loss have also been documented in the Trial of Nonpharmacological Interventions in the elderly (TONE) study and the Trial of hypertension prevention (TOHP) study [199, 200]. These studies suggest that weight loss of ∼2 kg is associated with 3.7/2.7 mmHg (systolic/diastolic blood pressure) reduction. The TOHP 2 study demonstrated that greater degree of weight loss, ∼4.4 kg, was associated with greater degree of blood pressure improvement, 5/7 mmHg [42]. The blood pressure reduction has been shown to be robust as long as the weight loss is sustained. Neter and colleagues’ meta-analysis of 25 studies that evaluated weight loss-dependent reduction in blood pressure suggested that on average each kg of weight loss resulted in ∼1 mmHg reduction in both systolic and diastolic blood pressure [201]. Achieving and sustaining weight loss remains one of the central strategies for blood pressure control in OH. While in some patients sufficient weight loss can obviate the need for antihypertensives in others it can reduce the number and doses of required antihypertensives [202].
The Place of Dietary Modification in OH Management
While there are many aspects of dietary modification that can have a salutary effect on blood pressure, the DASH (dietary approaches to stop hypertension) strategy is one of the most robust for which substantive prospective data is available [195]. The DASH diet is rich in fruits and vegetables as well as low fat dairy products [195]. It appears that the major mode of action of DASH in blood pressure amelioration is by induction of pressure natriuresis and diuresis [7].
Salt restriction is another well-validated lifestyle management strategy that is particularly relevant to OH. The DASH study showed that when salt restriction to ∼65 mmol/day in addition to the standard DASH program further improved blood pressure control [203–205]. It is now apparent though that the degree of responsivity to salt restriction is based on the salt sensitivity of the individual subject which appears to have strong genetic underpinnings [206]. The underlying basis for the salt-sensitive clinical phenotype is likely multifactorial and includes increased SNS activity, impaired response of the RAAS system and norepinephrine to sodium intake and volume depletion, suppression of the kallikrein–kinin system, and/or excess activity of the vasopressin pressor system [207]. It also appears that the salt-sensitive clinical phenotype most robustly identifies subjects with OH who respond best to weight-induced blood pressure improvement [207].
The Dietary intervention study in hypertension (DISH) was a prospective study that compared the weight loss achievement in three treatment groups: a weight reduction group, the sodium restriction group (daily intake of ∼40 mEq), and a nonintervention usual care group [208, 209]. The weight reduction group who had a caloric reduction counseling strategy implemented lost on average 4.5 kg at the 1-year follow-up time point and ∼60% of these subjects remained normotensive without requiring antihypertensive agents. This was in comparison to ∼30% remaining normotensive in the control group [208, 209].
The Place of Exercise in OH Management
The place of increased daily exercise in weight loss and moreso in effective weight management is well established [191–193]. It is also important beyond establishing a culture of regular exercise to ensure consistency and to also encourage a culture of reducing sedentary activities and increasing over all physical activity levels [210, 211]. Sustained increased physical activity is essential to long-term weight loss and is the best predictor of sustained weight loss [22]. Estimates suggest that regular aerobic exercise can reduce blood pressure by up to 11/6 mmHg though other data suggest more conservative reductions [212, 213]. Based on the most robust available clinical trial data the general clinical consensus is to recommend regular physical activity for at least 30 min daily for most days of the week. This has been demonstrated to not only positively impact blood pressure in OH but also ameliorate overall ASVD risk and also assist with redistribution of adiposity away from the visceral adipose compartment [192, 214, 215]. The salutary effects of exercise of OH appear irrespective of age, speed of walking, and have been estimated to reduce overall ASVD outcomes by ∼50% [192, 214–216]. It is also apparent from data and recommendations from the Institute of Medicine that for aerobic exercise “more is certainly better” as 60 min of moderately vigorous physical activity on a daily basis has been shown to impart even greater ASVD benefits including better blood pressure improvement and better impact on sustained weight management [217].
The Place of Alcohol Consumption Reduction in OH Management
Alcohol consumption is consistently associated with aggravation of hypertension and this is particularly relevant to OH as a lifestyle factor important for optimal blood pressure management. These findings have been replicated in multiple cross-sectional cohort studies [218]. Data suggests that constant daily consumption is associated with higher blood pressure than binge drinking [219–223]. Furthermore the degree of blood pressure elevation was not related to the type of alcoholic beverage taken [219–223]. Klatsky and colleagues suggest that the effect of alcohol on blood pressure has a “j” shaped curve so that alcohol abstainers tend to have higher blood pressures than those who imbibe between 1 and 3 drinks daily [222, 223]. It has been demonstrated prospectively that alcohol restriction does reduce blood pressure [224, 225]. The exact means by which alcohol elevates blood pressure is unclear but among the suggested mechanism are alterations in intracellular sodium flux, altered baroreflexes, altered intracellular calcium transport, magnesium deficiency (often nutritional related to poor caloric intake that often accompanies alcohol abuse), and altered insulin sensitivity [226]. Accepted clinical guidelines recommend alcohol restriction to no more than 20–30 g daily for men and 10–20 g daily for women in the general population. Whether even more stringent restrictions should apply to subjects with OH has not been directly studied.
Other Life Modification Strategies for OH Management
Behavior modification strategies and programs which have been used for decades in weight management of obese subjects if well deployed and sustained also have beneficial effects on blood pressure. Counseling that enables patients identify and address non-meal time eating cues is critical. In addition, encouraging subjects to keep a dietary intake inventory or food record helps with promoting self-monitoring and identification of deleterious eating patterns. Patients who can be convinced to keep detailed, consistent food records or diaries beyond providing valuable data to assist the dietician in prospective meal planning also often begin implementing appropriate behavioral change on their own volition [227]. Other behavioral modification strategies that may be useful in enabling weight loss as well as preventing weight regain include stimulus control, meal planning, stress and time management as well as deliberate reduction in sedentary time activities such as television watching and computer game playing.
Surgery and OH Management
Bariatric surgery is currently the most consistently effective weight loss strategy available for achieving sustained weight loss. Compared to other management modalities, its effectiveness is even more pronounced among subjects with severe obesity (BMIs ≥ 40 kg/m2) [228–230]. The Swedish Obese Subjects study (SOS) has some of most robust data demonstrating that bariatric surgery is associated with significant improvements in most of the metabolic complications of severe obesity including improvements in blood pressure levels [31, 32, 228–230].
Pharmacotherapy of OH
Because of the difficulties associated with achieving sustained weight loss though this may be the ideal means of managing OH, pharmacotherapy is necessary in the vast majority of patients with OH. Considering the multifactorial etiology of OH it is not unexpected that monotherapy for OH is often unsuccessful. Though there are no studies that suggest one class of antihypertensives being superior or preferred in OH. Management strategies designed at addressing the multiple pathogenetic pathways involved in the etiology of OH are preferred and most effective. Blockade of the RAAS, of aldosterone action, and of SNS overactivity appear to be the dominant pathways to address in achieving effective management of OH [7]. There have been relatively few clinical trials that have deliberately explored the unique management of OH [71, 231]and so most of the management recommendations are based on data gleaned from subgroup analysis of data from large prospective antihypertensive trials that include significant obese subpopulations. The ideal therapeutic regimen for OH is one that addresses the multiple anomalies associated with OH onset while protecting the heart, brain, and kidneys while improving or at least not worsening the metabolic milieu associated with OH. While there are no absolutely contraindicated or indicated antihypertensives for OH, patient regimens need to be individualized based on individual comorbidities, degree of hypertension, and dominant pathophysiologic features [192, 193, 232, 233].
The RAAS Modulators
Included in this broad subgroup of antihypertensives are the ACE inhibitors (ACEI), angiotensin receptor blockers (ARBS), renin inhibitors (RI), and the Aldosterone Antagonists (AA). These agents are generally quite useful in OH as it is often associated with RAAS activation, systemic aldosterone excess, and salt retention [73, 79, 80, 156, 183, 184]. As a group, these agents have several pleiotropic effects that go beyond their blood pressure reducing properties and these include reduction in glomerular hypertension, improving insulin sensitivity, reducing LVH, and reducing microalbuminuria [73, 79, 80, 156, 183, 184]. These group of agents overall should be included in any therapeutic regimen for a patient with OH unless there are absolute contraindications to their use. There are several prospective clinical trials that support this assertion including the TROPHY trial which was a prospective multicenter double blind trial in patients with OH comparing the ACEI lisinopril and hydrochlorothiazide [71]. The TROPHY trial did show that the ACEI was more efficacious among Caucasian and younger subjects as compared to African American subjects. Data from a cohort of obese hypertensive Japanese subjects showed that the ACEI enalapril has a better metabolic profile compared to amlodipine which included reduction in circulating insulin, leptin, and norepinephrine levels [234]. Telmisartan and Irbesartan are unique among the ARBs in benign shown to increase adiponectin tissue expression from adipocytes by inducing peroxisome-proliferator activated receptor (PPAR) gamma [235]. The major recent ACEI and ARB trials did not specifically enroll obese subjects though there were subsets of patients from all these trials who were obese. None of these studies showed the response of obese subjects to be inferior to their non-obese counterparts and thus it is reasonable to presume that the cardioprotective and renoprotective effects of ACEIs and ARBs demonstrated in such trials as HOPE, LIFE, VALUE, Micro-HOPE, and IDNT are applicable to subjects with OH [236–243].
The Diuretics
While the initial dominant effect of diuretics is on reducing intravascular and extravascular volume, over time the main antihypertensive mechanism for diuretics becomes reduction in peripheral vascular resistance [244]. Diuretics as a whole are generally used for short periods as monotherapy in OH and rather high doses are typically required [71, 245]. Furthermore these higher doses of diuretics are associated with a greater chance of worsening the various metabolic derangements often associated with obesity. As a result, diuretics are not generally considered as preferred agents for the management of OH in the setting of monotherapy though they do have a place as adjunctive therapy whether as second or third steps in the therapeutic regimen.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree