Other factors that contribute to weight loss in cancer patients include symptom distress and psychosocial factors, alterations in taste (dysgeusia), gastrointestinal dysfunction, and side effects of cancer therapy. The symptom-related and psychological factors associated with cancer that may alter food intake include pain, nausea, vomiting, anxiety, depression, and social isolation. Dysgeusia may be a direct side effect of chemotherapy, radiation therapy, and surgery, but it may also be psychological in origin (including food aversions and anticipatory nausea and vomiting).24,25 Gastrointestinal dysfunction is common in cancer patients; it may relate to direct effects of tumor (i.e., obstruction, compression of hollow viscuses), or to side effects or complications of therapy. Cancer surgery is invariably accompanied by a temporary catabolic state and decreased nutrient intake, and this may be prolonged by the development of complications such as obstruction, infection, and fistula formation. These may cause symptom distress, including alterations in taste, early satiety, pain, cramps, vomiting, diarrhea, and constipation. Chemotherapy often induces transient nausea and vomiting or injury to gastrointestinal mucosa with resultant stomatitis, mucositis, diarrhea, and/or typhlitis, all of which may be exacerbated by neutropenia. Radiation therapy can cause acute gastrointestinal injury accompanied by many of the previously mentioned symptoms and also chronic radiation enteritis with malabsorption and stricture formation.
The word “cachexia” comes from the Greek words kakos (bad) and hexis (condition). It is not surprising that the development of CCS confers a worse prognosis. The clinical features of CCS include weight loss, anorexia, fatigue, anemia, and hypoalbuminemia. Cancer cachexia can be present at any stage of disease, although it is more common with advanced malignancy. Weight loss is a central component of the undernutrition that occurs in cachectic patients. It varies between tumor types, and is most profound for tumors of the pancreas and the upper gastrointestinal tract.7
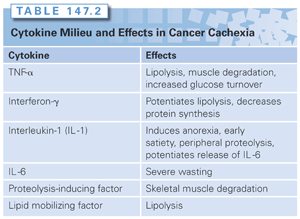
The etiology of CCS is multifactorial. Anorexia of malignancy is defined as a spontaneous decrease in food intake,26 and is most often mediated by cytokines.24 An abnormal metabolic rate; altered cellular metabolism of lipids, proteins, and carbohydrates; and a deranged cytokine milieu (Table 147.2) are the other causes of cancer cachexia. The interplay of these factors varies from patient to patient.
Energetics
Early studies that evaluated resting energy expenditure (REE) in cancer patients concluded that it is increased.27,28 However, these investigations did not account for fat-free mass (FFM) when calculating the REE. Weight-losing patients initially lose fat mass, and body composition should be accounted for when estimating REE.29,30 Even with these adjustments, inaccuracies still exist in the estimation of REE in cancer patients.31–33
The type of malignancy and the host response have been hypothesized to result in the variability of the metabolic rate that has been observed in cancer patients.31,32,34–39 For example, although patients with hepatobiliary neoplasms are mostly hypometabolic, gastric and lung cancer patients are frequently hypermetabolic.40–42 Factors such as increased acute phase reactants, prolonged duration of illness, and advanced cancer have been associated with an elevated REE, whereas the stage of the tumor has not.32,35–38 Additionally, because of anorexia and other factors, cancer patients may not adjust their food intake to compensate for the altered REE.26 The hallmark of REE in cancer patients is its variability and unpredictability.
Intermediary Metabolism
Increased turnover (cycling) of proteins and lipids is a feature of CCS, leading to decreased skeletal muscle mass and depleted fat stores. Augmented proteolysis and attenuated peripheral protein synthesis cause severe muscle loss in weight-losing patients with advanced cancer, which can result in respiratory complications and death.43,44 Proteolysis-inducing factor (PIF) is a major mediator of protein catabolism in cancer patients; it activates nuclear factor kappa B (NF-κB), which then turns on the ubiquitin-proteasome proteolytic pathway.20 By mass, most of the weight loss in cancer cachexia occurs from the depletion of fat stores. The primary mechanism behind this is elevated fat cell lipolysis. Similar to PIF, lipid-mobilizing factor (LMF) is central to the process of adipocytic lipolysis via regulation of hormone-sensitive lipase (HSL).43 Interestingly, both proteolysis and lipolysis are not suppressed with the administration of exogenous nitrogen45 and glucose.46 With respect to the metabolism of carbohydrates, multiple aberrations stem from the Warburg effect, an early 20th century observation that glycolysis is the main process for energy generation in cancer cells.47 This energy-inefficient process leads to an increase in hepatic gluconeogenesis and can cause the normal tissues to be energy starved.
Cytokines and Hormones
In CCS, many of the processes that have just been described are driven by an altered cytokine milieu (see Table 147.2). Both tumor cell production and host responses contribute to the deranged cytokine milieu.48
Although integral to local host defenses in the face of inflammation or infection, tumor necrosis factor-α (TNF-α) can have harmful systemic effects.49 These include muscle breakdown mediated by the ubiquitin-proteasome pathway and nitric oxide synthase expression, fat depletion that occurs because of increased lipolysis through the activation of HSL and decreased lipogenesis, and anorexia that is induced when TNF is produced or administered in the brain.50 Evidence for the role of TNF in cachexia comes from multiple animal models.51,52 Similar to TNF, interferon-γ (IFN-γ) is an important contributor to muscle wasting and fat burning through the activation of these same processes.53 Antibodies to these two key cytokines have been developed with some success in suppressing the metabolic changes of CCS, but no single agent has been able to prevent most of its features.49,53
Interleukin-1 (IL-1) and interleukin-6 (IL-6) are two proinflammatory cytokines that further contribute to CCS. IL-1 induces anorexia, increases peripheral breakdown of muscle, and promotes the release of IL-6.53,54 In turn, IL-6 has been demonstrated to elevate the rate of hepatic gluconeogenesis and peripheral proteolysis, and can result in more profound wasting than TNF.53,55 Despite the almost indistinguishable effects of TNF and IL-6, the latter (unlike TNF) is easily found in the serum of cancer patients with cachexia, and likely works through different mechanisms.53
Besides cytokines, several hormones and tumor-derived proteins have been implicated in CCS. These include leptin, serotonin, PIF, and LMF. Proteolysis-inducing factor and LMF are predominantly produced by tumor cells, whereas leptin and serotonin are generated by host tissues. PIF contributes to peripheral muscle wasting and to decreased protein synthesis, whereas LMF is central to lipolysis. Interestingly, LMF is detectable in weight-losing cancer patients, but not weight-stable cancer patients.50
The key function of leptin, an adipocyte-derived hormone, is to regulate body fat mass by reducing appetite and increasing REE.56 It does so via hypothalamic neuropeptides.48 When there is an increase in food intake, leptin levels are elevated. This leads to the suppression of appetite stimulants ghrelin and neuropeptide Y (NPY) and the stimulation of appetite suppressors corticotropin-releasing factor (CRF) and melanocortin. Persistently decreased levels of leptin have been identified in cachectic patients, indicating that tumors are capable of mimicking leptin and causing sustained anorexia and weight loss.48,55 Another hormone that has been found to contribute to the anorexia of cancer cachexia is serotonin.48 Several mechanisms have been proposed for this, including the depression of appetite and the inhibition of ghrelin secretion.57,58
The multiplicity of factors, both clinical and metabolic, that underlie CCS have, to date, frustrated most attempts to reverse its devastating clinical sequelae.
NUTRITION SCREENING AND ASSESSMENT
Nutrition screening to identify cancer patients at risk for malnutrition and a nutrition assessment to elucidate their nutrient requirements are the first step in the nutrition care of cancer patients.18 Objective parameters such as albumin, transferrin, and body weight changes are used in various formulae to predict preoperative risk in malnourished cancer patients (Table 147.3). The Prognostic Nutritional Index (PNI) has been validated prospectively and calculates the percentage risk of an operative complication in an individual. It can differentiate patients at low risk for nutrition-related complications (<10%) from those that are at high risk (>50%).59 The Nutrition Risk Index (NRI) was utilized to stratify nutrition risk in the Veterans Affairs Total Parenteral Nutrition Cooperative Study Group trial of perioperative parenteral nutrition and classifies individuals as either well nourished or malnourished.60 The Hospital Prognostic Index (HPI) identifies high-risk patients and evaluates the efficacy of hospital therapy.61
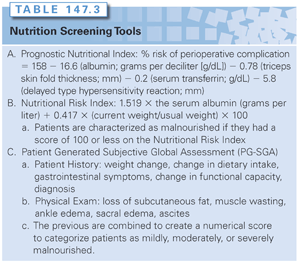
As effective, and more generalizable and practical, are various questionnaire-based tools; they can effectively and reproducibly categorize nutrition risk in cancer patients. The most frequently utilized and validated tool is the Patient Generated Subjective Global Assessment (PG-SGA). Prognostic factors such as weight loss, performance status, and nutrition-related symptoms are assessed in the PG-SGA. The clinician combines physical examination findings (especially loss of subcutaneous fat and the presence of muscle wasting, ankle and sacral edema, and ascites) with historical factors such as weight change, change in dietary intake, presence of gastrointestinal symptoms, functional capacity (performance status), and primary diagnosis to establish a global assessment of nutrition status (A: well nourished, B: moderately malnourished, C: severely malnourished). A numerical score can be added to the global rating creating the scored PG-SGA, which is the standard tool utilized by Oncology Dietetics Practice Group of the American Dietetics Association. The PG-SGA is practical, cost-effective, validated, and considered by some to be a gold standard.62
PHARMACOTHERAPY OF CANCER-ASSOCIATED WEIGHT LOSS AND MALNUTRITION
Cancer cachexia and the associated weight loss cause symptom distress and impair quality of life in cancer patients. They are associated with fatigue, anorexia, early satiety, and social isolation, as well as other symptoms.63,64 Furthermore, cancer treatments are often accompanied by gastrointestinal symptom distress relating either to the underlying cancer or, more generically, to the hormonal and cytokine milieu that accompanies the CCS. Qualitative research suggests an important role for psychosocial interventions directed at both the patient and family members to address these symptoms.65 Much of the focus, to date, however, has been on pharmacologic interventions.
Progestational agents have been the mainstay of the pharmacotherapy of cancer-associated anorexia and weight loss. Megestrol acetate and medroxyprogesterone acetate have been shown to improve appetite and to ameliorate weight loss in studies of patients with cancer and cachexia.66,67 Improved quality of life has been demonstrated in prospective studies in patients with cancer cachexia treated with megestrol acetate, with minimal side effects.68,69 Doses studied ranged from 160 to 1,280 mg per day, and maximal weight gain was generally seen within 8 weeks. The mechanism of action may, in part, be mediated via alterations in IL-6 levels.70 One study suggests that combining megestrol acetate with a cocktail of anti-inflammatory and antioxidant nutrients may have an additional benefit.71 Dronabinol, a marijuana derivative, has been shown to improve appetite and cause weight gain in small studies. Combined therapy with megestrol acetate dronabinol is not superior to megesterol acetate as a single agent.72
Anabolic steroids have no proven efficacy. In human trials, corticosteroids produce transient improvement in nutritional parameters and appetite, but continued use is associated with a negative nitrogen balance, net calcium loss, glucose intolerance, and immunosuppression.73 Agents such as growth hormone, insulin-like growth factor I, insulin, ghrelin, and melatonin, although promising in rodent models, have not been shown to be helpful in humans in improving nutrition status or quality of life.73–80 Cyproheptadine, an antihistamine and serotonin antagonist, can reduce diarrhea and reverse weight loss in patients with carcinoid syndrome.
Cytokine-directed therapies offer the promise of potentially reversing the cascade of metabolic and hormonal events that result in cancer cachexia. Animal studies are promising. However, although some agents have demonstrated effects in humans, none have been demonstrated to be clinically effective.18 Thalidomide and pentoxifylline have been shown to inhibit TNF-α, but have not demonstrated clear efficacy in clinical trials.81,82
Eicosapentanoic acid (EPA) and docosahexaenoic acid (DHA) are α-3 omega fatty acids found in high concentrations in fish oils that have anti-inflammatory properties.83,84 Unfortunately, they have not been shown to be effective in clinical trials in patients with cancer cachexia.84,85
Medications to relieve cancer- or treatment-related symptoms that impair oral intake can be important adjuvants to reduce or even eliminate the need for nutrition support in many cancer patients.86 Nausea, vomiting, diarrhea, and constipation are common. They can lead to an aversion to eating, with resultant weight loss. When these symptoms are present, a formal evaluation to look for an underlying cause and intervention by a registered dietitian has been shown to be helpful.87 General approaches include the use of antiemetics, promotility agents, antidiarrheals, fiber, laxatives, and (when pancreatic insufficiency is suspected) pancreatic enzyme replacement.88,89 Depression occurs in 25% to 45% of patients with cancer and can lead to a loss of appetite and weight loss.90,91 Antidepressant medications can help.90–93
NUTRITION SUPPORT OF CANCER PATIENTS
Because malnutrition is associated with a poor prognosis in cancer patients, it seems intuitive that nutrition support should improve cancer outcomes. Unfortunately, this notion is an oversimplification. The mere provision of nutrition support does not guarantee that the cancer patient will optimally utilize the nutrients to improve clinical outcomes. However, evidence does suggest that some form of nutrition support can be effective and can improve clinical outcomes in cancer patients who are currently undergoing anticancer treatment (surgery, chemotherapy, and/or radiation) AND who are moderately or severely malnourished OR who are anticipated to be unable to meet their nutrition needs orally for a period greater than 7 to 14 days in perioperative patients and greater than 14 days in nonsurgical patients.94 Guidelines that specify the cancer patients who are likely to benefit from nutrition support (i.e., the provision of nutrients other than orally) have been published by major nutrition organizations.94,95 Evidence-based guidelines published by the American Society for Parenteral and Enteral Nutrition for nutrition support during anticancer treatment and in hematopoietic cell transplantation are summarized in Table 147.4.

The routine use of nutrition support in cancer patients is not indicated. More than 35 clinical trials, some of them prospective and randomized, including more than 5,500 patients, have been summarized.96 In adequately nourished patients, nutrition support by the enteral or parenteral route does not improve outcomes in patients receiving chemotherapy or radiation therapy or who are undergoing cancer surgery, and in fact, nutrition support may actually harm these patients.96 Nutrition support should be reserved for those patients who are actively receiving anticancer treatment and who are malnourished or who are at high risk of becoming acutely malnourished because of an anticipated inability to take in adequate oral nutrition for 7 to 14 days or longer.
Optimal Route of Administration of Nutrition Support
On the initial presentation, dietary counseling and oral supplementation is often the best approach to support the nutrition needs of cancer patients.97–100 However, this may not be feasible secondary to side effects of cancer therapy, generalized cancer cachexia, abnormal physiology secondary to the malignancy, or anatomic factors that preclude oral intake. When oral supplementation is not an option, enteral nutrition (EN) is preferred. The enteral route is more physiologic, has fewer associated complications (especially infection), has a decreased incidence of associated hyperglycemia, and is more cost effective than parenteral nutrition (PN).101–103 EN can improve nitrogen balance and stimulate weight gain in cancer patients.104 EN is generally contraindicated in patients with bowel obstruction, abdominal distention, ileus, severe diarrhea, gastrointestinal bleeding, and high output external fistulas.
If use of the gastrointestinal tract is not feasible, then PN should be used. PN can facilitate weight gain; however, this is primarily in the form of fat.105 Historically, it was thought that the use of PN may mitigate morbidity and decrease mortality in the perioperative setting in gastrointestinal malignancy patients. However, many studies suggest that in adequately nourished patients, the perioperative use of PN in patients with gastrointestinal malignancies actually increases the incidence of infections and does not improve survival.94 However, severely malnourished patients can benefit because the use of PN in the perioperative setting in these patients can reduce the incidence of surgical complications and can improve survival in comparison to standard isotonic fluids.106
Optimal Timing for Initiation of Nutrition Support
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








