Abstract
There appears to a be an optimal window of nutritional sufficiency to allow for reproduction to occur in males and females. The male hypothalamic pituitary (HP) axis is relatively static and therefore is more resistant to nutritional perturbations than is the female HP axis. However, accumulating data indicate that both under- and overnutrition have consequences on the pace of sexual maturation in humans and on subsequent reproductive function. This chapter will review current knowledge of this field.
Keywords
Puberty, hypothalamus, pituitary, obesity, nutrition
In males, pulsatile gonadotropin-releasing hormone (GnRH) and gonadotropin secretion occurs at a mean interval of every 2 hours, a frequency sufficient to maintain testosterone secretion, normal virilization, and spermatogenesis. In females, a more complex series of gonadal tasks must be accomplished, which include maturation of a single follicle, follicular rupture and ovulation, and corpus luteum formation. The mature female hypothalamic-pituitary-ovarian (HPO) axis must respond dynamically with negative and then positive (bimodal) feedback to rising estradiol, which is secreted by the developing follicle. The positive feedback response, in the form of a luteinizing hormone (LH) surge, must be sufficient to initiate the molecular events of follicle rupture and subsequent luteinization. Optimal functioning of the female reproductive system requires more versatility of the HPO axis, and this system is therefore more vulnerable to disruption than is the male (see Chapters 1 , 12 , and 20 ).
Pulsatile secretion of hypothalamic GnRH and pituitary LH occurs prior to birth and continues throughout the prepubertal period. Pulsatile LH secretion has been detected in children and occurs at a normal, adult frequency in boys and girls ; however, the amplitude of the signal is miniscule and requires highly sensitive measurement methods to detect. Suppression of pulsatile GnRH-LH secretory amplitude in prepuberty has been attributed to a combination of enhanced sensitivity of the childhood reproductive axis to estradiol, as well as a predominance of inhibitory neural and neuroendocrine signals that ultimately dampen the amplitude of the central GnRH pulse generator. This working model hypothesizes that prepubertally, the HP axis is inhibited, and during puberty the axis is released from this inhibition.
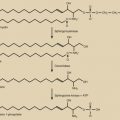
Over 40 years ago, Frisch and Revelle noted a direct relationship between body weight and age at onset of puberty and concluded that a critical amount of body fat was needed for the onset of puberty (see Chapter 17 ). These investigators predicted correctly that adipose tissue somehow provided a permissive signal to the reproductive system. The discovery of the endocrine nature of adipose tissue has since led to the identification of a number of adipokines as well as inflammatory factors, and the elucidation of their role in reproduction is emerging rapidly. The best characterized molecule to date is leptin. It has been shown in both animal models and in humans that inadequate leptin is associated with lack of GnRH secretion, and that leptin replacement can restore normal cycles in women who have hypothalamic amenorrhea and hypoleptinemia. However, leptin alone is insufficient to initiate pubertal maturation of the hypothalamic-pituitary-gonadal (HPG) axis and a specific leptin concentration or threshold, above which maturation occurs, has not been identified.
One of the final common pathways that appears to activate GnRH-LH secretion during puberty involves kisspeptin and its cognate receptor, G-protein coupled receptor-54 (GPR54). Kisspeptin appears to act directly on GnRH neurons and amplifies GnRH and consequently LH and follicle-stimulating hormone (FSH) secretion. Both kisspeptin and its receptor, GPR54, increase coincident with the onset of puberty. In addition to kisspeptin, there are many other upstream regulators of GnRH that appear to provide redundancy to prevent reproductive failure.
Puberty is also a time during which acquisition of functional networks of neurons and glial cells provide necessary communication pathways through which reproductive signaling can occur. The acquisition of these networks may involve anatomic changes in cell populations or recruitment as well as increases in neuronal connectivity. For example, glial cells long believed to be inert but helpful in providing scaffolding may play a more direct role in GnRH secretion. Microglia, macrophages that differentiate into glial cells, are necessary for optimal GnRH secretion. Colony-stimulating factor (CSF)-1 knockout mice, which lack adequate macrophage function, do not have adequate microglial development and are deficient in hypothalamic GnRH secretion. The interaction of immunomodulatory molecules with the reproductive neural networking is not yet well characterized.
Much research to date on abnormalities of pubertal maturation has focused on inadequate nutritional states. However, in the United States as in most developed nations, these conditions are becoming increasingly rare and are being replaced by reproductive dysfunction due to overweight and obesity. Obesity has been linked to altered pubertal progression and adult reproductive dysfunction. Polycystic ovary syndrome (PCOS) has been well studied in its interactions between obesity and anovulation; however, a far greater proportion of the population is affected by simple obesity. Pubertal onset is earlier in obese girls and boys, but the tempo of puberty is slowed in simple obesity. A relative state of hypogonadotropic hypogonadism has been described in obese women without PCOS. Decreased fecundity, increased reproductive wastage, and a less favorable response to fertility treatments have all been described in association with adult female obesity. In men, reduced fertility and a marked increase in bioavailable estrogen have been described.
This chapter discusses the role of overnutrition and undernutrition in relation to reproduction and provides emerging data from clinical trials designed to ameliorate the adverse effects of obesity on puberty and adult reproductive function.
Nutrition and Puberty
- ◆
Initiation of puberty likely represents a release from chronic inhibition of the HPG axis in both boys and girls.
- ◆
Low-amplitude hormone fluctuations may occur during the prepubertal period.
- ◆
Obesity may affect the timing of puberty by advancing its onset, but it may slow the tempo and have an inconsistent effect on the timing of menarche.
- ◆
The known and conjectured mechanisms initiating puberty are reviewed elsewhere in this volume. We will focus on the relevant epidemiology of normal puberty in boys and girls.
Puberty appears to be imminent when hypothalamic-pituitary sensitivity to negative feedback inhibition by estradiol is reduced, and attendant inhibitory neural networks diminish in activity. These processes lead to increased GnRH secretion as well as increased gonadotropin pulsatility, initially at nighttime, which then extends throughout 24 hours. The result is folliculogenesis in girls, spermatogenesis and testosterone production in boys, and the attainment of fertility milestones. In females, the ultimate maturation of the HPO axis is the manifestation of positive hypothalamic-pituitary feedback to increasing estradiol, which initiates an LH surge and ovulation of the preovulatory dominant follicle.
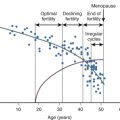
Sexual maturation is a progressive process, but it is not linear. Girls who have their first menstrual period do not always continue to ovulate monthly thereafter. It is also not certain if menarche routinely reflects an antecedent ovulatory cycle, or if it is merely indicative of a rise and fall in estradiol. Prior epidemiologic studies have observed that the attainment of regular menstruation takes from as little as 2 to as much as 7 years after menarche. One study of 112 Caucasian girls, followed every 6 months perimenarcheally, observed elevated urinary progesterone levels consistent with regular ovulation within 1 year of menarche in the majority. However, sampling was only done every 6 months, a frequency inadequate for the detection of monthly ovulatory events. Zhang and colleagues completed a study of perimenarcheal girls using daily urinary sampling, and the findings reinforce the notion that regular ovulation occurs relatively rapidly, within months instead of years after menarche. Metcalf and colleagues and Borsos and colleagues, who did weekly hormonal assessments in perimenarcheal girls, concluded that a mature pattern of ovulatory cycles was established in a majority of girls after the 20th menstrual period. However, even weekly urine sampling may be inadequate for the detection of brief excursions of progesterone, or its urinary metabolite, pregnanediol glucuronide (PDG). Luteal phases may also be shorter than the adult norm of 14 days when adolescents are observed. Data from our group indicates that the capacity to produce an ovulatory LH surge in response to estrogen is present even before the onset of the first menses. These findings indicate that, at least in some girls, a biphasic feedback response to estradiol is not the rate limiting step in attaining regular, ovulatory menses. Irregular menstruation has been associated with low gynecologic age, low body mass index (BMI), chronic nonspecific lung disease or allergic disease, weight loss, and stress.
Rosenfield has hypothesized that low-amplitude hormonal cycles occur in early puberty, with nighttime levels greater than daytime. Indeed, it may be that all the hormonal components of the HPO axis are functioning at an adult frequency, but the hormonal signals are dampened and slowly increase to detectable levels.
Prior epidemiologic studies documenting the age of attainment of regular menstruation were conducted in populations of girls who differ from our modern-day US population. There were few young women of color, yet African-American girls are known to attain menarche and to undergo pubertal maturation earlier than Caucasian girls. This has been related in part to increased BMI but may also be due to other factors. It is possible that prior populations studied have been enriched in girls who were nutritionally marginal, certainly so by today’s standards. However, weight or BMI data are not available from these earlier studies. Given the high rates of obesity among adolescents in the current US population, and the increase in body fat in adolescent women in the United States over the past century, it is likely that any restraint that low body weight might place upon the HPO axis is minimally operative in our current society. The virtual elimination of undernutrition might be expected to reduce the overall age at menarche in population studies. However, this is not uniformly the case. In one 50-year cohort and family study of a population sample in Ohio, higher BMI was not shown to be independently related to an earlier age at menarche. However, this population reflects minimal minority representation, and had a mean age at menarche of 12.7 prior to 1954 and 12.6 after 1954, a minimal decrease. It is possible that overall body composition or BMI in this sample was optimal prior to 1954 (i.e., few girls who were critically underweight were present in this sample). Data from the NHANES 1999–2004 examining age at first menarche in 6788 females over 20 years in the United States demonstrated a decline in the mean age at menarche from 13.3 years prior to 1920 to 12.4 years in those youth born between 1980 and 1984. While the downward trend was most notable for non-Hispanic black girls, significant declines in age at menarche were also documented among non-Hispanic white and Mexican-American youth. The association between BMI and age at first menarche was not examined in this study.
The notion that body weight or body fat is a permissive maturational signal to the hypothalamic-pituitary axis in girls is also supported by clinical data demonstrating an increase in leptin associated with puberty. Furthermore, within developing countries, pubertal timing variations exist between girls in well-off versus underprivileged conditions, with earlier ages at menarche in well-off girls. Utilizing data from the 1959–1962 National Health Examination survey (NHES) and the NHANES from 1971 through 1994, and 1999 through 2008, Krieger et al. evaluated the 50-year US trend in age at menarche among non-Hispanic black and white women and its association with socioeconomic status (SES) and race/ethnicity. While the average age of menarche declined in both groups over the 50-year time interval, white women in the lowest income percentiles had a higher average age at menarche than those in the highest income percentiles prior to 1962 with a reversal in this trend by 2005–2008 with lower average age at menarche in women in the lowest income percentile. In contrast, no socioeconomic trends were observed among black women over this time period. Over time, an increasing proportion of women with menarche at age less than 11 years occurred in both white and black women with lower SES. Although this study did not examine the association between BMI and age at first menarche, the overall declining age at menarche among both black and white women is likely due to improved nutrition associated with pre-1970 declines in childhood poverty followed by increasing adiposity among all socioeconomic strata and racial/ethnic groups. Additional research evaluating the interaction between, BMI, SES, as well as race and ethnicity is needed to explain these trends and to provide a more in-depth understanding of the societal and biological determinants of age at menarche. Whether these differences relate to nutritional status, physical activity or energy expenditure, or perceived environmental stressors related to income is not known. Environmental exposures are also hypothesized to contribute to the trend of earlier puberty in Western societies. Endocrine disruptors contained in plastics are ubiquitous in industrialized societies and most individuals demonstrate appreciable levels of exposure. In general, these compounds bind weakly to sex steroid receptors and their signaling is likely to be overridden by endogenous sex steroids in gonadally intact, reproductive aged males and females. However, at the extremes of reproductive life in women and prepubertally in men, these mixed sex steroid agonists may play a role in providing nonphysiologic input to the reproductive axis.
The known and conjectured mechanisms initiating puberty are reviewed elsewhere in this volume. We will focus on the relevant epidemiology of normal puberty in boys and girls.
How Does Fat Signal the HPO Axis?
- ◆
Leptin is a permissive signal to the HPG axis and appears to signal when nutritional stores are adequate for reproduction.
- ◆
Other adipokines may exert effects on the HPG axis, but their effects may be indirect and/or mediated by inflammation.
- ◆
There are compelling linkages between orexigenic peptide and sex steroid secretion in human and animal models.
Leptin and Its Effects on the Reproductive Axis
Leptin is a 16-kDa protein secreted primarily by adipocytes. Initially described as a satiety factor, it conveys an afferent signal to the central nervous system (CNS) about body fat stores. Mice deficient in leptin protein (ob/ob) or leptin receptor (db/db) exhibit hyperphagia, profound obesity, and central hypogonadotropic hypogonadism. It has been proposed that leptin exerts dual regulation of energy balance based upon the organism’s state of energy regulation. When energy input is equal to output, leptin concentrations reflect total body fat mass. Alternatively, in conditions of energy disequilibrium, leptin functions as a sensor of energy imbalance, with low concentrations in states of net weight loss and elevated in positive energy balance states (reviewed in ref. ). Leptin has been shown to modulate GnRH pulse frequency in vitro. It does not act directly on GnRH neurons, but rather via indirect mechanisms through interneurons secreting hypothalamic neuropeptides, such as neuropeptide Y (NPY), galanin-like protein, melanocyte stimulating hormone (MSH), and endogenous opioids.
Leptin’s role as a peripheral adipose signal to the central reproductive axis gave rise to speculation that it was the missing link in the Frisch hypothesis and, thus, served as a major trigger of pubertal development. Leptin’s role, while necessary, is insufficient to bring about pubertal maturation on its own. Leptin reverses pubertal arrest in leptin-deficient mice. When given to normal prepubertal animals, leptin hastens sexual development as manifested by the advancement of vaginal opening. However, when Cheung and colleagues assessed the temporal sequence of pubertal events in rodents, serum leptin was not detectably elevated prior to pubertal development. Moreover, expression of leptin receptor messenger ribonucleic acid (mRNA) in the hypothalami of female mice did not increase with pubertal development. Finally, administration of leptin to starved animals advanced estrus to the same degree as food-restricted untreated controls, but first estrus occurred at the same time as mice fed ad libitum. Taken together, these results imply that leptin provides a necessary input of adequacy of energy stores to the brain, thus authorizing but not initiating puberty.
In humans, leptin levels are markedly elevated in obesity and pregnancy and are overall higher in women than in men. Similar to rodents, deficiencies of leptin or leptin receptor, though rare, are characterized by early-onset obesity and variable degrees of hypogonadotropic hypogonadism. Dramatic reversal of pubertal delay with leptin administration in a leptin deficient girl has been described.
In the absence of a deficiency, serum leptin increases during childhood, with the highest concentrations in children who gain the most weight; higher serum leptin concentrations are associated with an earlier menarche. In boys with constitutional delay of puberty, a leptin rise was not required for pubertal progression. Furthermore, two women with lipoatrophic diabetes and chronic hypoleptinemia were reported to have normal menarche and childbearing, suggesting that normal pubertal maturation is possible even with very low serum levels. Thus while leptin does not act as a “trigger,” it somehow conveys an essential metabolic permission for the body to prepare for procreation. Women with amenorrhea of hypothalamic origin, with or without weight loss as a precipitating factor, demonstrate improvement of their menstrual cycles and restoration of regular, pulsatile GnRH-LH secretion in response to leptin. Leptin remains the most compelling molecule among currently known adipokines for having a major role in reproduction.
Leptin has numerous effects on peripheral components of the reproductive system in a variety of animals and model systems. On the gonadal level, leptin has been found in ovarian follicular fluid, and a leptin receptor has been localized to the human granulosa, theca, and Leydig cells. In a bovine model, leptin decreased gonadotropin-mediated sex steroid production from granulosa and theca cells. In humans, leptin may interrupt normal oocyte maturation and has been correlated with poor implantation potential. The predominant effect of leptin’s action on the HPG axis has been hypothesized to be dependent upon its concentration, whereby low leptin exerts a negative influence centrally, and elevated leptin yields a negative effect peripherally at the gonadal or embryo level.
Other Potential Molecules That Communicate Metabolic Signals to the Hypothalamic-Pituitary-Ovarian Axis
Adiponectin, another major adipokine, is the most abundant adipose gene transcript and is the natural peroxisome proliferator-activated receptor gamma (PPAR-gamma) ligand. It plays an overall protective role in the development of insulin resistance and, most notably, is profoundly decreased in obesity and type II diabetes. Adiponectin plasma concentrations reveal a sexual dimorphism, with adult females having significantly higher levels than males ; prepubertal children demonstrate no gender difference. Adiponectin levels significantly decrease after puberty ; however, it remains to be seen if this represents a consequence of the increasing body mass or is a distinct phenomenon.
Ghrelin, a 28-amino acid stomach-secreted peptide, is a ligand for the growth hormone secretagogue receptor. Ghrelin is a central appetite stimulator that regulates hunger and stimulates meal initiation and is another putative link between metabolic and reproductive axes. In cross-sectional studies, ghrelin concentrations decrease from childhood to adolescence, but children with central precocious puberty do not exhibit an increase in serum ghrelin after GnRH agonist treatment. In children evaluated for short stature, administration of exogenous sex steroids decreased serum ghrelin in boys but not girls. While one group related the fall in ghrelin levels to insulin-like growth factor (IGF)-I and IGF binding proteins as a putative mechanism for pubertal growth acceleration, larger longitudinal studies are needed to evaluate ghrelin’s role in pubertal development and reproduction.
Inflammatory cytokines, most notably tumor necrosis factor (TNF)-α and interleukin (IL)-6 among others, are produced by adipose tissue, and are elevated in obesity. In monkeys, leptin has been shown to modulate this inflammatory response. Weight loss results in a decrease in macrophage infiltration of adipose tissue and improvement of the cytokine profile. In obese adolescents, C-reactive protein is significantly elevated, providing the first indication that low-grade inflammation starts at a young age. It is not currently known whether mediation of this inflammatory response could mitigate some of the reproductive consequences of obesity. On the other hand, the hormonal status of the individual may be linked to the inflammatory cytokine profile. Recent studies have demonstrated an association between vasomotor symptoms and IL-8, implying that low estrogen levels somehow modulate expression of this cytokine.
NPY, a member of the pancreatic polypeptide family, is a 36-amino acid neurotransmitter predominantly found in sympathetic neurons. NPY is one of the most potent orexigenic peptides known and was recently shown to stimulate fat angiogenesis, proliferation, and differentiation when administered peripherally. Leptin decreases NPY gene transcription in the arcuate nucleus, thereby sending a signal to decrease food intake. In the male rhesus monkey, antagonism of an NPY receptor led to precocious GnRH release, suggesting a role for NPY in maintenance of the prepubertal brake on the GnRH pulse generator.
Sex Steroid Modulation of Feeding Behavior and Fat Accrual
The arcuate nucleus of the medial basal hypothalamus is a central relay station for body fat signaling and is a site of GnRH release. It functions to converge influences of orexigenic stimuli, such as NPY and agouti-related protein (AGRP), and anorexic stimuli, such as alpha-melanocyte-stimulating protein, corticotropin-releasing hormone (CRH), and opioids. In rats, exogenous leptin administration results in downregulation of NPY and AGRP. Expression of leptin receptors in the arcuate and ventromedial hypothalamic nuclei suggests this is a site of modulation of leptin effects on the reproductive axis. Recent evidence suggests that estrogen receptor (ER)-α expressing hypothalamic neurons regulate both feeding behavior and activity. Targeted deletion of ER-α in proopiomelanocortin (POMC) neurons leads to hyperphagia alone, but when steroidogenic factor-1 (SF-1) neurons undergo targeted deletion of ER-α, hypometabolism and hyperphagia can be created; deletion of ER-α in both of these neurons causes hyperphagia, hypometabolism, and abdominal adiposity. Moreover, ER-α deletion in these hypothalamic areas causes reproductive dysfunction. These data imply a critical and possibly developmental role for hypothalamic estrogen sensitivity and metabolism.
How Does Body Weight Modify Puberty and Adult Reproduction?
- ◆
Birth weight and the degree of “catch-up” growth that occurs can modulate the timing and tempo of puberty.
- ◆
Obesity is linked to earlier pubarche.
- ◆
Obesity advances the onset of puberty in girls, but lengthens its duration.
- ◆
Obesity delays and lengthens the pubertal process in boys.
Childhood Nutrition and Modification of Puberty
Reproductive maturation is delayed in a nutritionally deprived environment. The effect of obesity on pubertal timing has more recently been addressed in obese youth with premature pubarche (PP) in children with low birth weight (LBW) and rapid postnatal catch-up in weight, and in boys and girls with simple obesity. These conditions will be addressed in turn, as all inform the pathophysiology of nutritionally associated reproductive disorders in adults.
Effects of a Nutritionally Deprived Environment
Pugliese and colleagues evaluated nine boys and five girls, ages 9 to 17 years, with growth failure and delay in puberty (7 of the 14) due to malnutrition resulting from self-imposed caloric restriction. In these children, who restricted caloric intake for fear of becoming obese, increased linear growth and pubertal progression resumed with the resumption of age-appropriate caloric intake. Matejek and colleagues studied the relationship between leptin levels, fat stores, and reproductive hormone levels in 13 female juvenile elite gymnasts and 9 adolescent girls with anorexia nervosa. Leptin levels were subnormal and were related to body fat mass in girls with anorexia nervosa, but were lowest in the elite gymnasts. In both groups, estradiol levels were low and menarche was delayed. Catch-up height and weight in immigrant and adopted children, who move from developing to developed countries, is associated with precocious menarche. Lundeen et al. recently examined the association between early growth and the timing and tempo of puberty in predominantly black South African adolescents from low SES families in Soweto. Analyzing the anthropometric and pubertal data annually from age 9 through 16 years in 3273 children born in early 1990, the authors found both height and BMI in early childhood were positively associated with the tempo of puberty. The authors hypothesize that efforts to improve child health and growth in this area following the collapse of Apartheid may have contributed to a secular trend of lowered age of puberty over the study period. Taken together, these studies support the notion that a critical body fat mass is necessary for normal pubertal progression.
Effects of Low Birth Weight and Prematurity
LBW, small for gestational age (SGA) status, and prematurity are all associated with precocious pubarche (appearance of pubic hair earlier than 8 years of age) and early and exaggerated adrenarche in girls. Prenatal stress associated with prematurity may have an independent or additive effect on the programming resulting from growth restriction. LBW children have higher dehydroepiandrosterone (DHEA) levels compared to children of normal weight, indicating amplified adrenarche, and LBW has also been associated with an increased likelihood of developing PCOS. Although a majority of studies indicate that SGA is associated with PP and earlier onset of menarche, van Weissenbruch and colleagues observed no differences in the timing and progression of puberty, including age at menarche, between SGA and appropriate for gestational age (AGA) girls; another study found SGA to be associated with pubertal delay. More recently, Shim et al. observed lower adult height in girls born with SGA, yet birthweight was not associated with age at menarche. Studies associating birth weight with age at menarche have been similarly variable in outcomes, with findings ranging from no association to lower birth weight being linked to earlier pubertal onset.
Work by several investigators provides further insight into the progression of puberty in girls with precocious pubarche. Ibanez et al. documented differences in the timing of the onset of puberty and menarche dependent on the degree of prenatal growth restraint in 187 Northern Spanish girls followed from birth through adult stature. Girls with precocious puberty and LBW had menarche 8 to 10 months earlier and obtained an adult stature that was as much as 6.5 cm lower than non-LBW girls. While the LBW-PP girls demonstrated an accelerated onset of puberty and progression to adult stature, most of the height loss was observed prior to the onset of puberty and even before the development of PP. Low circulating levels of sex hormone binding globulin (SHBG; a marker for hyperinsulinemic insulin resistance in nondiabetic girls) and hyperleptinemia may contribute to the accelerated onset and progression of puberty in these LBW-PP girls. Severe pre- and postnatal growth restriction associated with insulin resistance and precocious puberty phenotype may be related to insulin-like growth factor 2 (IGF2) methylation abnormalities; however, further studies are needed to elucidate the exact mechanism for this relationship. The Dortmund Nutritional and Anthropometric Longitudinally Designed (DONALD) Study evaluated 215 children for the effects of early life exposures on the initiation of puberty. The authors found that children weighing between 2500 and 3000 g at birth initiated their growth spurt (defined as the age at minimal height velocity at the onset of the pubertal growth spurt) approximately 7 months earlier than children weighing more than 3000 g at birth. Children with LBW and rapid weight gain in the first 2 years of life displayed initiation of the growth spurt 4 months earlier than those with normal weight gain, indicating earlier pubertal onset. These children also had an earlier age at peak height velocity and earlier menarche in girls, indicating more advanced pubertal maturation. The associations were independent of prepubertal BMI, suggesting that other early life factors may influence the timing of puberty independent of the influences of body fat mass. Indeed the timing of postnatal weight gain is an important predictive factor for the age at menarche with earlier age at menarche more strongly associated with early postnatal weight gain in infants born with intrauterine growth restriction (IUGR). The findings relating to the relationship between birth weight and puberty initiation are variable and imply that there is not a simple, direct relationship. The variability may in part be explained by the different methods used for documenting pubertal initiation and progression: the use of Tanner staging, initiation of breast development, adrenarche, growth velocity, and initiation of growth spurt or menarche, as well as variability in the measures of assessing fat mass or body composition.
Effects of Simple Obesity in Girls
Longitudinal and cross-sectional data from studies conducted since the 1970s have demonstrated an overall close association between obesity and early pubarche in girls. The majority of members of an expert panel, recently convened to review studies conducted between 1940 and 1994 evaluating the secular trends of pubertal timing concluded that thelarche and menarche are occurring earlier in US girls. These trends coincide with the rise in obesity prevalence, leading to the hypothesis that increasing adiposity is associated with earlier pubertal development. Several authors have examined the relationship between obesity and early puberty in girls. In comparison with nonobese girls, obese girls have been found to have earlier menarche in Japan (9 months earlier) and Thailand (0.9 year earlier). Early menarche has also been associated with an increased risk of adult obesity, indicating that early menarche may precede the development of obesity. Yet other studies have shown that obesity precedes early menarche. Cooper and colleagues found that age at menarche was inversely associated with weight at 7 years, and He and Karlberg noted that incremental increases in BMI between the ages of 2 and 8 years were associated with early onset of the pubertal growth spurt in both boys and girls. The Fels Longitudinal Study examined the relationship between the mean age of menarche and the simultaneous change in BMI from age 3 to 35 years in successive birth cohorts (born from 1929–1990). While the age at menarche in the 1980s cohort was significantly lower than the age in girls born previously, and these findings match the findings of several recent reports, which suggests a decline in the mean age at menarche of US girls over the past 20 years, the decline in age at menarche was not associated with a significant concurrent increase in BMI in childhood or adolescence. In a longitudinal study evaluating the relationship between BMI and puberty onset in 354 girls, Lee and colleagues found that an increased BMI as early as age 3 as well as an increased BMI between the ages of 3 and 6 years was associated with an earlier onset of puberty. While these findings do not prove a causal effect, they suggest that increased adiposity precedes early puberty.
Precocious puberty has been linked with childhood obesity and girls with precocious pubarche tend to be hyperinsulinemeic and insulin resistant, especially if there is a history of LBW. Yet pubertal progression appears to be alternatively regulated in girls with simple obesity (i.e., obesity that is not complicated by premature adrenarche or PCOS). It is not clear whether early thelarche in overweight girls is related to central activation of the GnRH-gonadotropin axis or whether estrogens from other sources are responsible for the production of breast tissue. Increased estrogen production results from the peripheral conversion of androstenedione by aromatase contained in adipose tissue. Furthermore, the hyperinsulinemia that results in low levels of SHBG and higher levels of free circulating estrogen and androgens has been associated with excess total and central adiposity. Pharmacologic insulin sensitization appears to improve body composition and dyslipidemia and delays or prevents the progression to PCOS. These data favor an association between obesity and subsequent development of PCOS.
Although the onset of puberty in girls with obesity may be earlier, pubertal progression appears to be slowed in obese girls. The reasons for this are not completely elucidated; however, some recent work provides clues. In a cohort of 981 girls with idiopathic precocious puberty as well as a second cohort of 333 girls with central precocious puberty, a reduced LH response to exogenous GnRH was observed in association with increasing BMI. Similar to what has been observed in adults, obesity may exert restraint upon the reproductive axis by impairing pituitary responsiveness to endogenous GnRH. However, it remains unclear how obesity can simultaneously advance the onset of pubertal maturation while slowing the progression. Perhaps the increased availability of sex steroids, provided by excess adipose tissue, helps initiate puberty earlier.
Effects of Simple Obesity in Boys
In boys, studies evaluating the association of obesity with pubertal onset timing lack concurrence, with many studies reporting an association between obesity and later onset of puberty, whereas others report earlier onset of puberty associated with obesity. Utilizing Third National Health and Nutrition Examination Survey (NHANES III) survey data, Wang demonstrated that boys with a higher BMI were more likely to have a later, rather than earlier, sexual maturation. The accuracy of the ascertainment of genital stage in boys in the NHANES III has been called into question based on the observation by Herman-Giddens and colleagues that 25% of 8-year-old boys were at genital stage 2, whereas there had been no concurrent increase in the rate of boys being evaluated for precocious puberty (defined as the onset of testicular enlargement at <9 years). The early staging of puberty (without assessment of testicular volume) is subjective, and inadequate criteria for genital assessment may have resulted in an overclassification of prepubertal boys as having initiated puberty. Evaluation of pubertal onset utilizing testicular size to define pubertal onset has yielded variable results. Using orchidometry, Crocker et al. measured testicular volume on 439 predominantly African American and white boys and found a significantly smaller testicular volumes in obese boys compared to nonobese boys (7.5 vs. 9.2 mL) and concluded that a later onset of puberty in boys is associated with higher BMI. Interobserver correlations for pubertal assessments were not conducted in this study. In contrast, Fu et al. concluded that obesity may be associated with earlier pubertal onset by demonstrating an increased testicular size in obese prepubertal Chinese boys relative to nonobese age-matched boys (1.18 vs. 0.82 mL). Furthermore, morning inhibin B, DHEA, and DHEA-S and bone age were also higher in obese versus nonobese controls. In yet another study, Laron demonstrated that while obese Israeli boys were taller and their bone age was more advanced, at all ages, relative to age-matched controls, there was no difference between the two groups in terms of the onset and development of pubarche, voice change, testicular volume, and penile size. Recently, Lee et al. reanalyzed a large ethnically diverse ( n = 1931 white, n = 1000 African American, and n = 941 Hispanic) community-based pubertal dataset from the American Academy of Pediatrics’ Research in Office Settings study to evaluate the association between overweight and obesity and pubertal timing in US boys. Pubertal assessment was conducted by clinicians who were trained to measure testicular volume and genital and pubic hair Tanner staging. Interobserver reliability was ensured through the use of a training manual, a two-part qualifying exam prior to subject enrollment and the use of intraclass correlations for pubic hair (0.94) and genital development (0.83) assessment, respectively. The authors found that compared to normal-weight or obese boys, overweight boys experienced earlier puberty. In contrast, compared to normal-weight or overweight boys, obese boys experienced later puberty. The lack of concurrence of the various studies examining the association between obesity and pubertal onset may relate to variations in the methods for determining pubertal onset as well as significant differences in the different populations evaluated. Furthermore, assuming that adipose aromatase activity may result in excess production of estrogen in boys, the extra estrogen associated with obesity may suppress puberty initiation to an even greater extent in obese boys relative to overweight boys where at lower levels, it may propel the initiation of puberty. A majority of the studies do not distinguish between overweight and obese boys and so it is not clear to what extent the degree of overweight or obesity impacts the outcomes related to pubertal onset in these other studies.
Longitudinal studies have been conducted to further understand the association between obesity and pubertal onset and progression. In a 10-year longitudinal population-based study on 401 boys enrolled in the National Institute of Child Health and Human Development Study of Early Child Care and Youth Development, Lee and colleagues found that a higher BMI Z-score trajectory during early to middle childhood was associated with a later onset of puberty. A higher proportion (14%) of boys in the highest BMI trajectory were prepubertal (defined as a Tanner stage 1 determined by visual inspection) at 11.5 years old than those in the lowest BMI trajectory (7.7%), indicating a delay rather than advancement of puberty in obese boys. Vizmanos and colleagues, following 323 boys annually to assess pubertal onset at ages 11, 12, 13, or 14 years old, noted a positive relationship between BMI and age of pubertal onset (defined by testicular volume and genital development according to Tanner stage), yet significant differences in percentage body fat between the different ages at onset of puberty were not noted. These findings suggest that, similar to girls, boys require a critical fat mass to initiate pubertal maturation. Sorenson and colleagues conducted a cross-sectional study from 1991 to 1993 and a combined cross-sectional and longitudinal study from 2006 to 2008. Measuring pubertal onset based on testicular volume assessments and genital staging in healthy Danish boys, the authors found that age at pubertal onset declined by 3 months from 1991 to 2006 and that age at entry into genital and pubic hair stages 4 and 5 was reached significantly earlier in 2006 than 1991. In addition, BMI increased significantly during that time period. Importantly, earlier age at onset of pubertal testicular growth in 2006 compared with 1991 was no longer significant after adjustment for BMI—indicating that BMI at least partly explained these findings. Juul and colleagues studied pubertal onset in 826 boys and found that while taller compared to youth in 1964, the timing of pubertal onset between 1964 study and the 1991 to 1993 study had not changed. In another 10-year longitudinal study, Juul and colleagues utilized voice change as a marker for later pubertal development in 463 boys and found a downward trend in the age at voice break between 1994 and 2003. Furthermore, boys in the heaviest BMI quartile at 8 years of age had a 1.74-fold risk of early voice break relative to boys in the thinnest quartile. Tracking skeletal maturity and linear growth acceleration throughout infancy, childhood, and adolescence from birth through 18 years old in 521 subjects from the Fels Longitudinal study, Johnson and colleagues found that overweight or obese adults were more skeletally advanced throughout childhood. While BMI-related differences in peak height velocity were observed for both boys and girls, these differences decreased as puberty progressed so that by 18 years old, overweight or obese adults were not significantly taller than their normal-weight peers. More recently, Song et al. used four cross-sectional Chinese National Surveys on Students’ Constitution and Health (1995, 2000, 2005, and 2010) to examine the relationship between spermarche and BMI. Over the 15-year time interval, the investigators documented a drop in the age of spermarche from 14.57 to 14.03 years and an association between higher BMI and lower age at onset of reaching spermarche was observed. In a subsequent study using the same datasets, a similar trend documenting a decline in the age at spermarche in a majority of 11 ethnic minority groups from 1995 to 2010, as well as an association between higher BMI and lower age at onset of reaching spermarche was observed indicating a positive association between higher BMI and earlier onset of pubertal initiation. Worldwide, even in longitudinal studies, the relationship between obesity and timing of puberty appears to differ by the manner in which markers of puberty as well as the definition of overweight and obesity are defined and documented. In addition, ethnic and racial variations likely contribute to these observed differences.
Research on the physiology of puberty in obese girls and boys may help explain these apparent discrepancies. Bordini and colleagues examined nighttime LH pulsatility in 40 pubertal girls and observed blunted LH secretion in girls with a body weight greater than the 85th percentile. These investigators hypothesize that there is a bimodal female HPO axis response to androgens. As described previously, adrenal androgen excess is linked to premature adrenarche and early onset of puberty. This process seems to occur in girls whether or not they are destined to develop PCOS. However, once puberty is initiated in non-PCOS related obesity, the relative hypogonadotropic hypogonadism of obesity retards the process, leading to a normal onset of menarche. The research focus on menarche as an easily measurable physiologic end point of reproductive maturation may be obscuring the pathophysiologic alterations in the stepwise process of puberty in girls.
Adult Obesity and Reproduction
- ◆
Obesity adversely affects reproduction on many levels of the HPG axis.
- ◆
There are two obesity phenotypes in women: hyperandrogenic obesity associated with PCOS and “simple” obesity without polycystic ovaries.
- ◆
Obesity is associated with an increased risk for preeclampsia.
- ◆
Obesity is associated with relative hypogonadotropism.
- ◆
This defect appears to be related to pituitary insensitivity to normal GnRH secretion.
- ◆
Similar to females, male obesity is associated with relative hypogonadotropic hypogonadism.
- ◆
Only the more severe obese phenotypes appear to be associated with reduced fertility in men.
- ◆
Weight loss appears to mitigate obesity related reproductive dysfunction.
- ◆
More clinical trials are needed to define the optimal amount of weight loss needed and the appropriate time of post weight loss recovery needed prior to attempting to conceive.
Genetic Predisposition to Simple Obesity: Reproductive Correlates
The latest NHANES study indicates that over 35% of all US adults are obese, defined as BMI of greater than 30 kg/m 2 . By 2015, 75% of all US adults are projected to be overweight or obese with BMI of at least 25 kg/m 2 . Perhaps even more concerning are the recent data on US women born in the 1980s who were found to exhibit up to 21% higher propensity to large body mass when compared to women born in the 1960s. Thus further escalation of the current obesity rates is likely as the 1980s generation ages and reaches the peak obesity prevalence. Easy availability of high-calorie nutrition and low physical activity have been implicated as potential causes for these trends, yet there are few definite answers as to why the obesity epidemic is continuing unabated.
Individuals respond differently to the environmental obesogenic challenges. In twin studies, up to 60% to 70% of total variation in percentage of body fat has been attributed to genetic contribution. In 2007, one genome-wide association study has identified multiple single nucleotide polymorphisms (SNPs) in a gene designated Fat mass and obesity-related transcript (FTO) as mediators of the relationship between large body mass and type II diabetes mellitus. This breakthrough in understanding of genetic contribution in patients with simple or nonsyndromic obesity has been replicated by other groups with an underlying theme that specific FTO variants correlate with human obesity. Up to 16% of adults who are homozygous carriers for the risk of FTO allele were reported to weigh 3 kg more and have up to 70% increased risk of obesity when compared with noncarriers. The FTO gene was first reported in 1999 in a fused toe (Ft) mouse model. Some of the deleted genes in this model had no known function at the time, and one of these genes was serendipitously termed “Fatso (FTO)” because of the large size of the gene. Subsequent studies have shown that FTO primarily functions as a single stranded RNA demethylase and is upregulated in rodent hypothalamus after food deprivation. While genes that predispose to adiposity may mediate impaired energy expenditure, FTO genes appear to be important in regulating food intake. In human studies, subjects with some FTO risk alleles were found to have diminished satiety awareness and loss of eating control.
PCOS (see Chapter 21 ) is the most common endocrinopathy affecting up to 10% of all reproductive age women. It is frequently accompanied by obesity with some studies reporting up to 50% prevalence in PCOS women. Obesity appears to modulate the risk of PCOS. Studies from Asian populations with lower adiposity as compared to non-Asians typically report fewer affected subjects, as exemplified by 2.2% PCOS prevalence in a cohort of 915 Chinese women presenting for an annual physical. Similarly, one Spanish study reported 28.3% prevalence of PCOS among consecutively studied obese or overweight women, which was markedly higher than the 5.5% prevalence reported among lean women by the same investigators. A recent study of Polish women suggested that FTO risk alleles may portend a greater impact in women with PCOS as compared to general population. In this study of 136 young women with PCOS, a 10-kg difference in weight was found between the carriers and noncarriers of the FTO risk allele, a difference that is 2 to 3 times greater than what was reported for the population at large. A recent meta-analysis from 7 studies and 2548 PCOS women has confirmed this trend. This novel relationship between FTO inheritance and PCOS may reflect either an interaction between FTO physiology and environment or multifactorial and polygenic nature of PCOS. The take home message here is that FTO may affect obesity in PCOS or PCOS may, in turn, amplify the effects of FTO on adiposity. While a more complete picture of all genes linked with adiposity and reproductive competence is still emerging, it is imperative to emphasize that the recognition that these exist is important for understanding why some women are particularly vulnerable to obesity and its reproductive consequences.
Women
Adult obesity in women is associated with two reproductive phenotypes. In the presence of marked hyperandrogenism, women who are genetically vulnerable, including those with the newly identified FTO risk alleles, can develop PCOS in association with weight gain. This notion is implied by abundant data on the salutary effects of weight loss on fertility in anovulatory women with PCOS. However, PCOS is only present in 5% to 7% of the adult population, and the current population prevalence of obesity in reproductive aged women is much higher. Therefore a second pathway must be hypothesized to explain the reproductive phenotype in simple obesity that is unrelated to PCOS. As PCOS will be covered in great detail elsewhere in this volume, this discussion will center on the model for reproductive dysfunction in women with simple obesity.
Increased body size is associated with reduced fecundity in both women and men. Women who are overweight and obese in their late teens are almost 4 times more likely never to conceive compared to normal-weight women. If they do conceive, obese women experience increased reproductive wastage and worse outcomes with assisted reproductive technologies than do normal-weight women. In 162 patients undergoing in vitro fertilization (IVF) at one center, obese women required more gonadotropin, yet had lower numbers of oocytes retrieved and 45% lower fertilization rates compared to women of normal weight. Fedorcsak and colleagues reported similar findings in a sample of over 5000 IVF cycles in 2660 couples. Cumulative live births were 10% lower (41.4% vs. 50.3%) in women with a BMI over 30 kg/m 2 compared to women with BMIs between 18.5 and 24.9 kg/m 2 along with increased gonadotropin requirements and reduced oocytes retrieved. Others have reported similar detrimental outcomes for obese women undergoing assisted reproductive technologies (ART). Among 1293 women who underwent IVF at one US center, women with a BMI >40 kg/m 2 had more than double the cancelation rate of normal-weight women (and more than triple the cancelation rate when PCOS was not present) and a linear trend towards higher risks of preeclampsia and cesarean delivery.
Obesity and Preeclampsia
Preeclampsia complicates 5% to 7% of pregnancies and maternal obesity is a consistent risk factor for preeclampsia; however, the mechanisms involved are not understood. The dose-dependent relationship between increasing BMI and the risk of developing preeclampsia is well established. For instance, an elevated BMI results in an odds ratio of 7.2 for developing preeclampsia as opposed to normal BMI women. The relationship between obesity and risk of preeclampsia has been observed in several populations throughout the world, suggesting that this phenomenon is not limited to western cultures. Furthermore, increased BMI in the normal range is also associated with an increased risk of preeclampsia. Fat mass is likely an important contributor, which is supported by the observation that weight loss reduces preeclampsia risk. Obesity and preeclampsia are both associated with oxidative stress and circulating markers of inflammation. Plasma levels of C-reactive protein and inflammatory cytokines, TNF-α, IL-6, and IL-8 are elevated in obese individuals and preeclamptic women. In addition, obese and preeclamptic women often have comorbidities of dyslipidemia, insulin resistance, and impaired endothelial function. These shared features suggest that preexisting inflammation may be the link between obesity and preeclampsia.
Relative Hypogonadotropic Hypogonadism of Obesity
The relative hypogonadotropic hypogonadism of obesity partially explains the fertility impairments. Sherman and Korenman reported longer cycle length, longer follicular phase length, and reduced daily LH, FSH, and progesterone secretion in association with hirsutism in five ovulatory midreproductive-aged women who were overweight or obese compared to a larger sample of normal-weight women. Grenman and colleagues compared 25 women of very high BMI (mean weight = 120 kg) to 25 normal-weight controls and observed decreased estradiol (E2), SHBG, androstenedione, and LH in the obese. These changes were partially reversed by acute, modest weight loss (mean loss of 13.2 kg). A number of investigators have reported decreased inhibin B in association with increased weight, along with reduced follicular phase gonadotropins and E2. The reduced follicular phase gonadotropin and luteal phase progesterone secretion observed in women of large body size can be attributed to relatively insufficient FSH stimulation of folliculogenesis, a model that has been well described in nonhuman primates.
Studies to date in animals and humans consistently point to a defect in LH pulse amplitude in association with obesity. In obese, leptin-resistant Zucker rats—a model similar to human obesity—there is profound suppression of pulsatile LH amplitude but not frequency. It seems likely that some consequence of excess nutrient sensing plays a role in the reproductive dysfunction associated with obesity. Jain and colleagues have observed a dramatically reduced amplitude of endogenous LH pulses in women with a BMI greater than 35 kg/m 2 , implying a central defect in reproductive function that may in turn impair ovarian and endometrial function. Women awaiting bariatric surgery who underwent frequent blood sampling had mean LH pulse amplitude of 0.87± 0.1 compared with 1.59 ± 0.15 IU/L in normal-weight controls. Post surgery, after at least 25% of initial body weight had been lost and weight had stabilized, there was a partial return of LH pulsatility and menstrual cycle parameters to those of normal-weight controls.
Both inhibin B and müllerian inhibiting substance (MIS) or anti-müllerian hormone (AMH) are reduced in obese women, despite the lower overall sex steroid secretion throughout the menstrual cycle. These data indicate that FSH is inappropriately low for the given inhibin B levels in obese women. The fact that inhibin B is suppressed in obesity is interesting, because inhibin B is widely regarded as a marker of ovarian reserve. However, obese women do not have an earlier menopause. The dual suppression of both MIS and inhibin B in obesity indicates that factors other than low ovarian reserve are operative, perhaps at the level of the ovary.
There is emerging evidence of impaired pituitary responsiveness to GnRH as a mechanism that underlies the relative hypogonadotropic hypogonadism of obesity. Obese women given a physiologic bolus of 75 ng/kg of GnRH demonstrate an impaired LH and FSH response. Normal-weight women administered an infusion of lipid in the setting of a hyperinsulinemic, euglycemic clamp—thus reproducing the excess free fatty acid and hyperinsulinemia of obesity—demonstrated a reduction in circulating LH and FSH. These findings suggest that circulating factors associated with the obese phenotype may influence pituitary secretion of LH and FSH.
Mice that experience dietary-induced obesity (DIO) have hypogonadotropic hypogonadism, and animals who are resistant to DIO-related reproductive dysfunction have reduced central inflammation. Recent studies demonstrating the ability of melanocortin to block proinflammatory cytokine or peptide-mediated LH suppression suggest a potential mechanism by which pituitary output of LH and FSH are inhibited in obesity. Thus the emerging relationship between inflammation, nutrient sensing, and reproduction may provide further clues to explain the reproductive phenotype of obese women.
Men
In contrast to the substantial body of literature on the impact of female obesity on reproduction, the corresponding effect of male body mass on the ability to reproduce is only recently coming to the forefront of investigative attention. Obese women with an overweight or obese male partner have up to twofold further loss in fertility as compared to their obese counterparts with normal-weight male partners. A dual loss of fertility is more likely, however, because overweight and obese women tend to marry or cohabit with men of similar weight. At the population level, several large observational studies have recently demonstrated an inverse relationship between male obesity and fecundity. Data from US farmers indicated that each 3-unit rise of male BMI was associated with a 12% increase in prevalent infertility in affected couples. Similarly, Danish National Birth Cohort records indicated a dose-response relationship between male adiposity and involuntarily delayed pregnancy. Furthermore, data from over 26,000 Norwegian pregnancies revealed that the detrimental influence of male obesity on fertility was demonstrated even when coital frequency data was included in the analysis, suggesting that this effect was not mediated by sexual dysfunction. More recently, adiposity of the male partner has been associated with decreased chance for clinical pregnancy after ART.
Male obesity may affect spermatogenesis and has been linked with reduced semen parameters. A study of over 500 infertile couples from Utah has shown that large body mass tripled the relative risk of oligospermia and reduced total motile count in men with BMI over 30 kg/m 2 . Similar associations were reported in healthy young Danish men reporting for a prearmy physical and Egyptian infertility patients. In further support of these observations, increased DNA fragmentation has been demonstrated in sperm from obese men. Recently, a meta-analysis based on data from over 9700 men from 14 studies worldwide demonstrated that, as compared to men of normal BMI, both overweight and obese men were more likely to have oligospermia and azoospermia. Remarkably, men with BMI over 30 kg/m 2 had approximately 40% higher chance of presenting with oligospermia and up to 80% higher chance of presenting with azoospermia. While the precise mechanisms underlying these associations remain uncertain, adiposity leads to reproductive hormonal changes that may alter spermatogenesis.
Similar to obese women, decreased LH pulse amplitude but unaffected LH pulse frequency have been reported in obese men. The endocrine profile of hypogonadotropic hypogonadism in obese men leads to significantly depressed testosterone and is often, but not always, associated with erectile dysfunction. A dose-response relationship between hypoandrogenism and the degree of obesity has also been reported and is thought to occur in concert with a corresponding increase in circulating estrogens. On the pathophysiologic level, obstructive sleep apnea (OSA), commonly diagnosed in obese men, has been linked with reproductive hormone alterations. In 1989, Grunstein and colleagues demonstrated reduced overnight secretion of testosterone in obese men with OSA. Treatment of OSA has been demonstrated to result in the increase of serum testosterone with either surgical (uvulopalatal resection) or medical (nasal continuous positive airway pressure, nCPAP) management. In obese men with OSA, pituitary gonadotropins have been demonstrated to be significantly reduced as compared with normal-weight controls and partially reversed by correction of hypoxia. Taken together, the potentially reversible hypogonadism associated with OSA and the decreased fertility and semen parameters associated with male obesity make a strong case to explore the mechanistic implications of symptomatic sleep disruption on sperm production. This is especially relevant given the recently shown significant improvement of male fertility with antioxidants and findings in the murine diet-induced obesity model of de novo induced oxidative stress, sperm DNA damage, and decreased fertilizing ability.
Impact of Weight Loss and Bariatric Surgery on Reproductive Function
In normal-weight women, weight loss of as little as 10% to 15% reverses deficits in LH pulsatility. In overweight and obese women, small improvements of fertility have been reported with diet-achieved moderate weight loss in some studies. Increased regularity of menstrual cycles and improved fertility has been reported in women who maintain weight loss. However, a more recent controlled trial of 574 obese, infertile women randomized to a lifestyle intervention that resulted in a mean weight loss of 4.4 kg demonstrated a reduction of the live birth rate compared to controls of 0.77 (95% confidence interval 0.6 to 0.99). On the other hand, a pilot trial of lifestyle modification including caloric restriction, weight loss medication, and exercise in women with PCOS who deferred treatment with clomiphene citrate to accomplish the weight loss indicated that ovulation rates were markedly improved after the lifestyle intervention. Additional trials with live birth as the outcome are needed to truly determine the ability of weight loss to improve and reverse the fertility deficits associated with obesity. It is important to quantitate the benefit, because behavioral weight reduction is notoriously difficult to achieve and notoriously reversible when dieting is not aggressively maintained.
Bariatric surgery produces dramatic and long-lasting weight loss with reductions up to 60% of presurgical body weight. Most bariatric patients experience an initial, dramatic weight reduction of 3 to 4 pounds per week for the first 6 months, followed by a plateau phase within a year. The chief mechanism for weight loss is thought to be consumption of smaller meals due to the decreased volume of the stomach. In women who have undergone bariatric surgery, improvement in menstrual irregularities and higher fertility rates have been reported. Most of the literature support a favorable risk-benefit ratio in support of bariatric surgery in patients with a BMI greater than 40 kg/m 2 . In men, with the exception of one case report of unexplained spermatogenic arrest after bariatric surgery-induced weight loss, there is a paucity of studies assessing the impact of weight loss on male reproductive success, assisted or unassisted. In small case series, obese men showed an overall improved reproductive hormone profile following weight loss, with an increase in SHBG and testosterone and decrease in estrogen after bariatric surgery. Given the increasing utilization of bariatric surgery for morbid obesity as well as recently advocated indication for type II diabetes mellitus, comprehensive studies on the impact of surgical loss on male and female reproductive potential are urgently needed.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree