and Julia Challinor6
(5)
Department of Hematology/Oncology, Boston Children’s Hospital, 300 Longwood Avenue, Boston, MA 02115, USA
(6)
School of Nursing, University of California, San Francisco, 2 Koret Way #N-611Y, San Francisco, CA 94143, USA
Overview
Childhood cancer care requires a multidisciplinary team of highly trained professionals to ensure safe and effective care along the treatment continuum. Nurses represent the largest workforce in health care, and are uniquely positioned to evaluate patient and family response to disease and treatment in the context of the social and cultural environment [1].
In low-and mid-income countries (LMIC), high nurse–patient ratios and the absence of specialized oncology nurse training programs are factors that contribute to suboptimal outcomes [2, 3]. Multiple robust studies have shown that low nurse staffing is associated with increased mortality and adverse patient outcomes, highlighting the need to match staffing with patient needs for nursing care [4]. Increasing the numbers of specialized nurses has also been shown to contribute to increased survival rates [5]. However, local schools of nursing or hospitals in LMIC often do not recognize the need for pediatric oncology specialization [6]. Nurses in LMIC must rely on short-term visiting teaching programs, or establish partnerships with expert nurses through twinning programs, to compensate for the lack of formalized training.
Collaborative relationships, or twinning, between nurses in resource-rich centers and those in resource poor settings is a successful model for providing education and mentoring to pediatric oncology nurses. St. Jude Children’s Research Hospital developed a twinning approach which has been successful in many resource poor countries; this model has been replicated by cancer institutes and foundations throughout the world [7, 8]. Twinning for nursing involves the selection of a nurse educator in the resource-limited setting who collaborates with a nurse leader from the partnering site. Nurses in partnerships must prioritize the retention of specialized nurses and the development of pediatric oncology nursing specialty programs in local nursing schools or hospitals, where the culture and priorities of the country are embedded into the program [3, 9, 10]. Cost-effective, local innovations built into educational programs have been found to be effective in LMIC [11].
Along with shortages of technology and sufficient supplies to carry out nursing responsibilities [12, 13], lack of respect and low prestige for nurses within health care teams are also substantial obstacles to optimal nursing care in LMIC [14–16]. Opportunities for nursing leadership and networking on an international level are few for nurses in LMIC. However, Steward and Usher noted, “Empowerment of nursing leaders and managers, increased focus on the patient….exploring conditions for frontline nurses and the direct relationship between improved nursing conditions and increased patient safety [in developing countries] mirrors literature from developed countries” [17].
Pediatric oncology nurses are involved in all aspects of cancer care, including early detection, physical and psychosocial care throughout treatment, management of disease and treatment-related side effects, long-term survival, and palliative therapy. Although cancer care settings vary in the availability of resources and technology, pediatric oncology-specific education and training for nurses is necessary to ensure the provision of quality patient care. This chapter will outline the essential elements of pediatric oncology nursing which are applicable in low-, middle-, and high-income settings.
Standards of Care
To ensure the delivery of safe, effective, and family-centered nursing care, standards for nursing practice are essential. The Association of Pediatric Hematology/Oncology Nurses (APHON) published the updated “Pediatric Oncology Nursing: Scope & Standards of Nursing Practice” in 2007 [18]. APHON defines the highest standards for care, education, and professional practice. Joint Commission International standards have also been successfully adapted to guide pediatric oncology nursing standards in resource-limited settings [19]. Nursing leadership must be involved in decision-making around the appropriate standards for pediatric oncology nursing in a given setting, taking into account the availability of resources and the nature of the care environment.
Effective pediatric oncology care incorporates guidelines for nursing assessment, diagnosis, outcomes identification, planning, and implementation. Principles for communication and coordination of care, along with guidelines for patient and family teaching and health promotion, are essential. Nurses are expected to evaluate the child and family’s progress toward optimal health outcomes (Table 13.1).
Table 13.1
Pediatric oncology nursing standards of practice
Nursing process | Standards of practice |
|---|---|
Assessment | The pediatric oncology nurse collects and documents data regarding the child and family to guide the development of an appropriate plan of care |
Diagnosis | The pediatric oncology nurse uses assessment data from nursing and other disciplines to identify problems and determine diagnoses and appropriate interventions |
Outcomes identification | The pediatric oncology nurse identifies expected and desired outcomes specific to the patient and family. These include adequate education, optimal growth and development, physical and emotional health, minimal suffering and distress due to symptoms of disease and treatment, and optimal quality of life for dying children |
Planning | The pediatric oncology nurse develops an individualized plan that prescribes interventions to attain expected outcomes |
Implementation | The pediatric oncology nurse implements the plan of care to achieve the expected outcomes for the child and family, with the goal of improving the child’s health, promoting quality of life, and facilitating optimal family functioning |
Coordination of care | The pediatric oncology nurse coordinates the delivery of care to support transition across the continuum of care and promotes optimal communication among caregivers |
Health teaching and health promotion | The pediatric oncology nurse employs strategies to educate families about maintaining health and providing a safe environment of care |
Evaluation | The pediatric oncology nurse evaluates the child and family’s progress toward attainment of expected outcomes |
Standards for Professional Performance
Professional performance standards address competency, promotion of quality practice, and collaboration with patients, families, and the medical team. Inherent in professional performance is recognition of the rights of children and families to participate in decision-making in accordance with ethical principles, the integration of research into clinical practice, and management of a safe, effective care environment that it is mindful of resource utilization. Nurses also serve as leaders, mentors, and educators through modeling professional performance in nursing practice (Table 13.2).
Table 13.2
Pediatric oncology nursing: standards of professional performance
Standard of practice | Description |
|---|---|
Quality of practice | The nurse participates in activities that improve the quality, safety, and effectiveness of nursing care in all settings |
Education | The pediatric oncology nurse demonstrates competency in pediatric oncology nursing practice and maintains current knowledge gained from publications, research findings, and professional activities |
Professional practice evaluation | The pediatric oncology nurse evaluates own nursing practice in relation to professional practice standards, relevant statutes, and regulations |
Collegiality | The pediatric oncology nurse interacts with and contributes to professional development of peers, colleagues, and others |
Collaboration | The pediatric oncology nurse collaborates with children, families, and multidisciplinary team members in providing care |
Ethics | The pediatric oncology nurse respects the rights of all children and families and makes decisions and designs interventions that are in agreement with ethical principles |
Research | The pediatric oncology nurse contributes to nursing through participation, review, and integration of research |
Standards for Nursing Education
Ideally, nurses new to pediatric oncology will receive formal training to develop a sufficient knowledge base and clinical skills, and ongoing education will be provided. Although the needs will vary depending on the availability of resources and technology, basic content should include an overview of growth and development, review of common pediatric cancers, safe handling and administration of chemotherapy, assessment and management of pain and distressing symptoms, venous access management, nutritional assessment and management, and infection control and prevention. Nurses should also learn to recognize the early signs of sepsis and other oncologic emergencies. Instruction in assessing and managing the psychosocial, emotional, and spiritual aspects of cancer care, including supporting patients and families at end of life, helps to provide nurses with skills to manage the myriad of emotions that families experience during the cancer journey. If the creation of a nurse education program is not possible, the presence of standard policies and procedures that are available to nurses on the ward should be prioritized to promote safe, consistent patient care.
Pediatric oncology nursing involves caring for children with cancer across a continuum, from diagnosis to cure or a peaceful death. Nurses orchestrate multiple aspects of care and advocate for effective patient and family education, communication, and quality care. Nurses may also act to increase early diagnosis of cancer through public awareness, by promoting local cancer treatment options and partnering with parent groups and other nonprofit organizations in their countries to spread the message that childhood cancer is treatable and often curable [20].
Symptom Management and Supportive Care
Lisa Morrissey7 and Julia Challinor8
(7)
Department of Hematology/Oncology, Boston Children’s Hospital, 300 Longwood Avenue, Boston, MA 02115, USA
(8)
School of Nursing, University of California, San Francisco, 2 Koret Way #N-611Y, San Francisco, CA 94143, USA
Bone Marrow Suppression
Bone marrow suppression, or myelosuppression, is a common and potentially life-threatening complication of cancer and cancer treatment in children and adolescents. Bone marrow function may be impaired both by the disease process and chemotherapy, interfering with healthy production of white blood cells (WBCs), red blood cells (RBCs), and platelets. Complications include the increased risk of infection due to a reduction in the circulating number of neutrophils, anemia from decreased RBCs necessary for tissue and organ oxygenation, and an increased risk of bleeding due to diminished platelets that are required for blood clotting. Recognition and prompt response to the complications of myelosuppression is essential [21].
Anemia and Thrombocytopenia
Packed RBC transfusions at 10–15 mL/kg of body weight may be given if symptoms of anemia are present, such as pallor, tachycardia, fatigue, or shortness of breath. Platelet transfusions may be needed for patients who are severely thrombocytopenic or who are at high risk for bleeding due to infection, surgical procedures, or the presence of intracranial tumors.
Assurance that the donor blood supply has been appropriately screened for pathogens such as hepatitis, HIV, cytomegalovirus, and bacteria is essential to minimize transfusion risks. Nurses are responsible for monitoring complications of blood product support such as transfusion reaction or hypersensitivity and to monitor symptoms such as fever, chills, rigors, urticaria, hemolytic reactions, and volume overload [21]. A transfusion policy which provides guidelines for safe verification and administration of blood products, including vital sign parameters and standard procedures for transfusion reactions, is essential in patient care settings where transfusions are administered.
Fever and Neutropenia (F&N)
There are numerous causes of fever in neutropenic patients, yet the risk of severe bacterial infection makes rapid detection and urgent intervention essential [22]. The Infectious Diseases Society of America defines fever as a single oral temperature of ≥38.3 °C (101 °F) or a temperature of ≥38.0 °C (100 °F) over a 1-h period [23]. Axillary temperatures are not considered as an accurate measure of core temperature and rectal temperatures are discouraged to prevent colonizing gut organisms from entering the surrounding mucosa and soft tissues. Neutropenia is defined as a neutrophil count of less than 500 cells/mm3 or a count of 1,000 cells/mm3 with a predicted decrease to 500 cells/mm3 in the next 48 h. The risk of serious bacterial infection increases when the ANC is ≤500 cells/mm3 [24]. Management includes prompt, thorough evaluation for evidence of infection, initiation of broad-spectrum intravenous antibiotics, and hospitalization until the neutrophil count has sufficiently recovered. The risk for infection and infection-related mortality increases with the duration and severity of neutropenia. Other risk factors include the presence of central venous access devices, impaired skin or mucosal integrity, active disease with bone marrow involvement, use of corticosteroids, and asplenia [25].
Nursing management: A thorough nursing history is completed upon admission and a complete assessment is conducted daily throughout the course of hospitalization. A nursing physical exam should include assessment of the skin, oral mucosa, lungs, and perineum. Signs of infection include fever, oral lesions, redness at the site of tubes and lines (i.e., central venous access devices, gastrostomy tubes, and chest tubes), skin lesions, perirectal irritation or abscess, cough, rhinorrhea, ear or throat pain, and diarrhea [26]. Timely communication to the medical team of new or persistent fever or other infectious symptoms is essential.
Patient and family education: Caregivers must demonstrate understanding that fever in the setting of neutropenia is an emergency and can signal a life-threatening infection. Upon discharge from the hospital, they must provide reliable contact information and confirm that transportation to an emergency room is available. Written information with instructions on when and how to contact the doctor is recommended. Caregivers should be instructed to call the doctor before administering any fever-reducing medicine at home, and to be aware of the signs and symptoms of infection, including fever, fatigue, body aches, shaking chills, cough or shortness of breath, redness or swelling at the site of an injury or tube site, belly pain, mouth sores, diarrhea or rectal pain, and dizziness [27]. Special consideration must be given to children who live in rural areas or who do not have access to transportation to a local hospital or emergency room. Alternate arrangements should be considered during predicted episodes of neutropenia to ensure that access to medical care is available in the event of a fever.
Infection Control
The nurse is responsible for promotion of good hand washing and serves as a role model for infection control by thorough demonstration of hand sanitation techniques for the patients, families, visitors, and other health care personnel on the unit. If water, soap, and a disposable towel are not available, the use of alcohol-based hand sanitizer is recommended [28]. It is essential that nurses demonstrate and observe good hand washing with newly diagnosed patients and families so the crucial role of hand washing in preventing the spread of infection is understood [29]. The nurse must also be vigilant about the presence of an adequate supply of hand washing/sanitizing materials in the patient care area to promote proper hand hygiene by clinicians, family members, and visitors [27]. Children and adolescents may arrive on the unit with contagious infections. Adequate measures must be taken to isolate patients who potentially contagious, particularly in crowded settings where bathrooms are shared or plumbing are suboptimal. In addition, visitors should be screened for symptoms of infections conditions, such as fever, cough, runny nose, sore throat, diarrhea, skin rashes, or open wounds. Other preventative strategies include prohibiting the use of rectal thermometers or suppositories, educating the patient and family to avoid contact with people who are ill, and encouraging good mouth care and daily hygiene.
Gastrointestinal Complications
Gastrointestinal (GI) disruption is a common complication of cancer therapy. Mucosal cells divide rapidly, so are particularly sensitive to chemotherapy and radiation. Compromise of the mucosal barriers increases the risk of infection, dehydration, pain, bleeding, and malnutrition.
Mucositis is the inflammation of the mucous membranes and is a common side effect of chemotherapy. It can be mild to severe, and may be dose-limiting, particularly in children receiving high dose, intensive chemotherapy, radiation of the head and neck, or undergoing bone marrow transplant. Stomatitis refers specifically to an inflammation of the oral mucosa. Clinical manifestations include redness, edema, and ulceration of the mucus membranes, gums, and tongue. Symptoms include pain, changes in taste, cracked lips, and sore throat.
Nursing interventions include assessment of the oral cavity at least daily, pain assessment, promotion of good oral hygiene (including use of a soft toothbrush and toothpaste), and evaluation of oral intake and hydration status [29]. Topical agents or systemic analgesics ranging from acetaminophen to continuous intravenous narcotics are often prescribed to minimize pain until mucositis resolves. In some studies, the use of topically applied vitamin E has been found to be helpful to reduce or prevent mucositis [30]. Coconut water, applied topically, has also been reported to relieve mucositis as it promotes wound healing [31].
Constipation is noted as a decrease in the frequency of bowel movements, or hard, dry stools, often accompanied by abdominal discomfort, straining, rectal pain, or cramping. Constipation is often related to decreased gastric motility due to medications (especially vinca alkaloids and narcotics) or may also be caused by tumor compression, electrolyte imbalance, decreased physical activity, changes in appetite, and disruption of normal routine due to hospitalization. Laxatives and stool softeners are often used as prophylaxis against constipation. Drinking warm tea or prune juice and increasing dietary fiber (beans, cereals, and whole-grain crackers) can also help address constipation [32]. It is also important to stay well hydrated by drinking fluids and water throughout the day.
Nursing assessment includes close monitoring of frequency, volume, and consistency of stool output. Report any abnormal signs, such as bleeding or perirectal pain or fissure to the medical team. Interventions include promotion of adequate hydration and a rigorous bowel regime, such as a diet high in fiber and administration of preventative stool softeners or laxatives if constipation is expected before chemotherapy, such as with repeated doses of vincristine [33].
Nausea and vomiting are common and highly distressing side effects of chemotherapy. Acute nausea and vomiting typically begins within several hours of chemotherapy administration and resolves within 24–48 h. However, delayed symptoms can last up to 2 weeks. Poorly controlled nausea may result in complications such as dehydration, poor nutritional intake, electrolyte imbalance, and severe discomfort. Once the cycle of nausea and vomiting has begun, it is difficult to manage. Therefore, prevention before symptoms begin is the most effective approach. Premedication for children who either have a history of nausea or vomiting or who will receive known highly emetic chemotherapy is highly recommended.
Several drug classes of antiemetics are effective. Serotonin receptor antagonists, such as ondansetron and granisetron are highly effective, yet may be limited in availability due to cost. Dopamine antagonists, such as prochlorperazine (Compazine), and glucocorticoids such as dexamethasone, may also be useful in preventing symptoms before chemotherapy is administered. Metaclopramide with diphenhydramine is an effective combination that is often readily available, yet requires monitoring for the risk of dystonic reaction. Nurses are responsible to know the action and side effects of antiemetics and to monitor patients for hyperemesis and dehydration, reporting symptoms to the medical team [33].
Diarrhea is an increase in the quantity, frequency, or fluid content of stool that differs from the usual bowel pattern. Chemotherapy, bowel surgery, radiation therapy, and infection may all be causes of diarrhea [33]. Complications include dehydration, malnutrition, intestinal infection, electrolyte imbalance, and social withdrawal due to disrupted daily routines. Children with severe diarrhea must be closely monitored for signs and symptoms of dehydration and electrolyte imbalance. Nurses must track intake and output carefully and report imbalances to the medical team. Daily weights and careful monitoring of abdominal symptoms and perirectal breakdown are usually indicated. Patients with diarrhea should be isolated until infectious causes can be ruled out. Dietary measures to control diarrhea include eating small frequent low fat snacks, limiting milk products, and eating food at room temperature [32].
Nutritional Support
Providing children with food and nourishment is a fundamental parental task. In the hospital setting, nourishment may become a difficult issue. Children and adolescents may refuse to eat unfamiliar foods; in addition, chemotherapy and other treatments may change a child’s taste sensations turning formerly favorite foods to unpleasant or unusual tasting. Many treatment-related factors may impact the child/adolescent’s nutritional status, such as type of tumor, nausea/vomiting, constipation, and oral and gastrointestinal toxicities of medications [34].
Children in countries with limited resources may already be malnourished when diagnosed with cancer, although obesity is on the rise and may also impact nutritional status [35]. Poor oral intake increases the risk of dehydration, infection, and gastrointestinal symptoms such as constipation. In settings where food is scarce, the nurse must be particularly observant about what the patient is eating since parents and visitors may also have limited food supplies. Careful evaluation, monitoring, and documentation of the patient’s nutritional status are important upon admission and throughout treatment. It may be necessary to consider supplementing the child’s diet, particularly during induction therapy or in malnourished children with insufficient protein intake. Options may include oral intake of a high-calorie diet with additional protein (such as adding one egg a day or supplements such as Plumpy Nut), nasogastric enteral feeds, or total parenteral nutrition [36, 37].
Parents must be educated about safe storage and handling of foods during cancer treatment, such as washing fruits and vegetables before eating. They should be advised to avoid bringing food prepared under potentially unhygienic conditions into the hospital. Food preparation areas on the pediatric oncology unit stocked with snacks, juice, and bottled water are helpful to parents who want to supplement the child’s hospital diet. Parents are the experts on their child’s food preferences; partnering with parents to address nutritional issues in the hospital will enhance the child’s dietary health [38].
Pain Management
Pain is one of the most frightening and debilitating consequences of childhood cancer. Uncontrolled pain can result in decreased physical activity, poor appetite, fear, loneliness, depression, isolation, and mistrust of the medical team [38]. Pediatric oncology nurses are critical in the assessment, treatment, and evaluation of pain in children with cancer.
Clinical Presentation
Pain can be acute or chronic, or both, and may be the result of tissue damage or nerve injury. Pain originating in the bones, joints, muscles, skin, tissue, or viscera is usually receptive to pain medication, such as non-opioids and opioids. Neuropathic pain is the result of nerve injury and is often described as burning or shooting pain. Neuropathic pain is usually more difficult to manage, as it is less responsive to opioids and non-opioids [39].
Nursing Assessment and Intervention
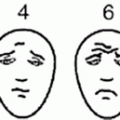
Pain assessment must take into consideration the patient’s developmental level and ability to express pain (Table 13.3). Pain evaluation ideally is completed routinely by nursing as the “fifth vital sign,” using a validated pain scale such as the Wong-Baker faces scale for younger children (Fig. 13.1), the numeric scale for older children, or the FLACC scale for nonverbal children.
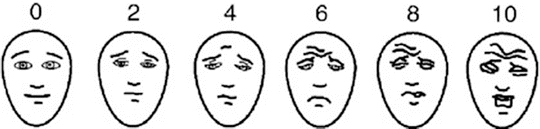
Table 13.3
Pain assessment by developmental level
Developmental level | Expression of pain |
|---|---|
Infant | Intense crying, inconsolable, draws his or her knees to the chest, hypersensitivity or irritability, unable to eat or sleep |
Toddler | Verbally aggressive, regressive behavior or withdrawal, guarding of painful areas |
Preschooler | May verbalize the intensity of the pain, view the pain as a punishment, and understand that there can be a secondary gain associated with the pain |
School-age child | Verbalizes pain, can use an objective measurement of pain, resists movement. Can be influenced by cultural beliefs and can experience nightmares associated with pain |
Adolescent | Can verbalize pain but may not request pain medications or may deny pain in the presence of peers. May experience changes in sleep patterns and in appetite or may display regressive behavior in the presence of family members |

Fig. 13.1
Wong-Baker Faces Pain Rating Scale
Pain location, quality, and intensity, along with medications or non-pharmacological interventions and subsequent response must be communicated to the medical team and documented appropriately by the nurse. Ongoing evaluation of pain and symptom relief is essential to quality patient care.
Chemotherapy Administration
Lisa Morrissey9 and Julia Challinor10
(9)
Department of Hematology/Oncology, Boston Children’s Hospital, 300 Longwood Avenue, Boston, MA 02115, USA
(10)
School of Nursing, University of California, San Francisco, 2 Koret Way #N-611Y, San Francisco, CA 94143, USA
Chemotherapy Safety
Safe administration of chemotherapy is an essential aspect of caring for children, adolescents, and young adults with cancer. The health care team shares responsibility in assuring patient safety by adhering to error prevention practices throughout the chemotherapy process. Pediatric oncology nurses have an important and active role in the patient’s pretreatment evaluation, the administration of chemotherapy and supportive care, as well as post-chemotherapy monitoring. The complexity of chemotherapy and the risk of adverse effects make a process for safe and effective administration of chemotherapeutic agents essential [40]. Ideally, a pharmacist should prepare all chemotherapy under a chemical hood with ventilation systems designed to reduce exposure and protect against toxic fumes. Awareness of the need for trained pharmacists is growing and should be strongly advocated for by pediatric oncology care teams [41].
When a nurse is responsible for preparing chemotherapy, training in chemotherapy preparation and personal safety is necessary. Along with a properly installed, ventilated biosafety hood, adequate personal protection equipment (PPE), which includes chemo safe gowns, disposable gloves, and protective eye gear are critical to minimize personal risk [42]. Adequate staffing of nurses per shift when chemotherapy is being prepared and administered is also essential for patient and nurse safety [43]. The allocation of a designated chemotherapy preparation nurse has been found to improve efficiency, reduce costs, minimize the risk of medication errors, and promote safety for both patients and nurses. The nurse preparing the chemotherapy can serve as the double check to the nurse administering the chemotherapy by verifying the patient name, route, dose, date, and time of chemotherapy medications [44].
Chemotherapeutic agents are administered in a variety of settings, including inpatient units, ambulatory clinics, and in the home. All nurses who administer chemotherapy must be educated about chemotherapeutic agents, the route of administration, side effects, and safe handling practices. A process for assessing the nurses’ knowledge and competency of chemotherapy administration is imperative before they are allowed to administer chemotherapy to a patient.
Chemotherapy Administration Process
Pretreatment assessment of the chemotherapy administration process starts with a patient assessment (Table 13.4) including past medical history, allergies, hospitalizations, and current medications. If the patient has received chemotherapy or radiation therapy in the past, it is important to assess for previous use and effectiveness of antiemetics, presence of side effects, and overall tolerability of treatment. This allows the nurse to determine if adjustments should be made for the current chemotherapy administration.
Table 13.4
Components of the pre-chemotherapy treatment assessment
Components | Comments |
|---|---|
Obtain and document baseline vital signs and pain assessment | Use validated pain tool for assessment |
Evaluate past medical history (PMH) | |
Evaluate current health history | • Recent illnesses |
• Current medications | |
• Use of complementary and alternative therapies | |
• Allergies (drugs, foods, products [i.e., latex]) | |
• Immunization status | |
• Nutritional status | |
Obtain height and weight | For initial chemotherapy treatment, obtain two unique heights and weights |
Remeasure height and weight before each chemotherapy cycle | |
Notify physician if >5–10 % difference since previous measurement | |
Assess need for rehydration | Dependent on chemo agents to be administered, yet twice maintenance is the standard used [51] |
Review orders, consult with prescribing MD | |
Calculate maintenance fluid using body weight method | • 4 mL/kg for the first 10 kg of a patient’s weight |
• 2 mL/kg for the next 10 kg of a patient’s weight | |
• 1 mL/kg for the rest of the patient’s weight | |
Example: Maintenance for 32 kg patient: | |
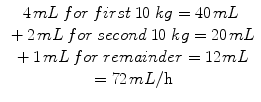 | |
Review patient’s previous chemotherapy experience (if any) | Focus on side effects and effectiveness of the supportive care regime. |
Ensure adequate fluid status by evaluating patient’s ability to tolerate oral fluids or receive IV hydration. | Monitor fluid status by assessing: |
• Skin turgor | |
• Fluid intake and urine output | |
Families must be instructed to save urine in urinals or bottles to ensure that urine output is at 1–2 mL/kg/h |
Chemotherapy Orders and Treatment Plan Review
Chemotherapeutic agents have a narrow therapeutic index and are considered high-risk medications, meaning there is a small difference between the desired treatment dose and a dose that can cause severe or fatal complications. For high-risk medications, an independent dose calculation by each chemotherapy competent clinician who prescribes, dispenses, and administers chemotherapy has been shown to reduce and prevent errors [45].
The administration of chemotherapy requires two clinicians to independently verify the correct patient and medication. When checking the medication, confirm the dose, route, scheduled administration time, correct fluid/volume, and compatibility. Ensure informed consent for treatment has been obtained and is available for review. The essential elements of chemotherapy orders include the following [5]:
Patient name and unique patient identifiers (such as birth date and medical record number)
Date and time order is written with the intended start date
Patient diagnosis
Protocol name and number (if applicable), treatment regimen, cycle, week, and day
Criteria based on treatment and specific patient information
Height (documented in centimeters), weight (documented in kilograms), body surface area (BSA), or other variables (age) necessary to determine dosage
The generic name of the drug (abbreviations are not acceptable)
Dosage of all agents in the regimen are clearly written (i.e., milligrams or grams, or units) as well as dosing parameters (i.e., ___mg/m2 = ___mg)
Leading zeros are acceptable (i.e., 0.4 rather than 0.4)
Chemotherapy diluent, volume, and rate
Number of doses to be administered, treatment duration, including start and stop date, total amount of drug administered per course and if applicable, cumulative doses
Supportive care medications necessary for treatment
Monitoring parameters (i.e., vital signs, intake and output requirement, when to notify the physician)
Other safety measures to incorporate into chemotherapy orders include prohibiting verbal chemotherapy orders, avoiding hand written orders, and requiring written documentation for any adjustment(s) to the order.
Chemotherapy Dosing Considerations
Pediatric chemotherapy drug doses are determined based on BSA in milligrams per meter square (mg/m2) or milligrams per kilogram (mg/kg). The formula to calculate BSA is:
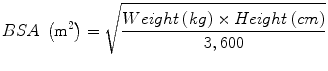
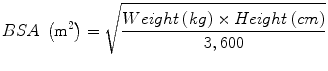
Children under 12 months or less than 6 kg are often dosed using mg/kg formula [46]. Dose per m2/30 is typically used, unless otherwise specified in the protocol or treatment plan. The dosing for intrathecal and intra-Ommaya chemotherapy is determined by the patient’s age [47].
Dose reductions may be made based on organ toxicity or toxicity from prior therapy. Agents, such as doxorubicin, (Adriamycin), dactinomycin (Actinomycin-D), the taxanes, docetaxel and paclitaxel, and gemcitabine (an antimetabolite), are dose-reduced or stopped during radiation therapy to prevent radiation recall. Symptoms of radiation recall may range from a mild rash or desquamation to painful, edematous vesicles or papules. Delaying the time between completion of radiotherapy and the start of chemotherapy can decrease the risk of radiation recall [48]. Lack of evidence exists supporting the need for adjusted dosing in obese patients compared with non-obese patients [49]. Dose escalation maybe protocol-specific or made if the absolute neutrophil count or platelet count does not decrease as expected.
Chemotherapy Administration
To safely administer chemotherapy, a designated chemotherapy preparation area away from any food or beverages must be identified. The chemotherapy drugs are brought to this area in a sealed plastic bag with a sticker labeled as “cytotoxic” in order to ensure proper handling by others [45]. This area must be equipped with PPE, chemotherapy hazardous waste container, and a chemotherapy spill kit. Ideally, all chemotherapy infusions are administered via an infusion pump with Luer Lock tubing connections [50]. Before administering chemotherapy and/or supportive care medications, review the medication label for the name of the drug, dose, and expiration date. Inspect the medications for discoloration and particulate matter. The last step is for two chemotherapy-competent clinicians to compare the chemotherapy product to the chemotherapy order, checking patient identification, name of the drug, route, dose, and correct infusion rate [51].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree