(1)
Nuclear Medicine Service, Hospital “Caduti Bollatesi”, Bollate (Milan), Italy
(2)
Department of Vascular Surgery, Center for Vascular Malformations “Stefan Belov”, Clinical Institute Humanitas “Mater Domini”, Castellanza (Varese), Italy
Keywords
angioscintigraphywhole body blood pool scintigraphylymphoscintigraphymagnetic resonancelymphographyIntroduction
Nuclear imaging technique may have a more important role in the evaluation of angiodysplastic malformations, where tissue structure subversion, unpredictability of the morphological picture, frequent multifocality, and, in a few cases, the remarkable extent of the corporeal districts cannot be unequivocally answered with the use of more traditional methods.
The frequent lack of a recognized anatomic and structural consideration and the difficult distinction between masses generically defined as “liquid” may result in ambiguous nuclear magnetic resonance (NMR) patterns, while the presence of an anomalous escape course, segregated vascular districts, low recirculation speed, or the pathological mixture typical of lymphovenous abnormalities represents an impasse for angiographic interpretation.
In this corner of “diagnostic half-light,” nuclear investigations make a useful contribution, albeit with the intrinsic limitations of a discipline that found its methodology more on functional than on morphological aspects [1].
While there are some procedures that have been made obsolete by the technical and diagnostic refining of radiological studies, the nuclear methods that have resisted represent a real complementary diagnostic tool for the evaluation of angiodysplasia, thanks to their procedural simplicity, lack of invasiveness, high tolerability, and low biological cost from a dosimetric point of view [2].
Whole-Body Blood Pool Scintigraphy (WBBPS)
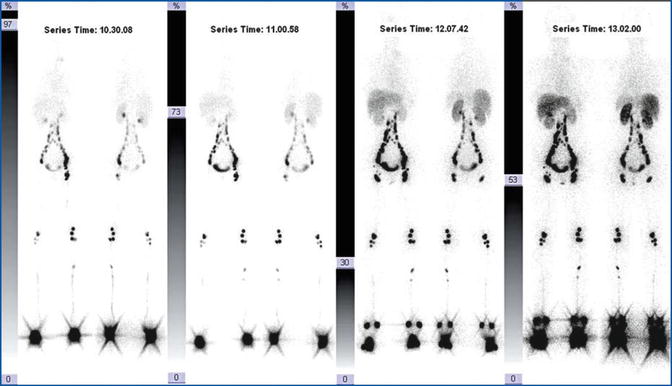
WBBPS (Fig. 28.1) is valuable for the initial screening of malformations and as an intermediate evaluation parameter for partial corrective procedures (post-interventional and/or post-embolization control). On the other hand, therapeutic or conservative treatments must be attempted several times in the instrumental follow-up of suspicious recidivism. WBBPS, a simple procedure from a technical point of view, makes red blood cells visible through the use of physiological contrast medium. This allows the vascular tree to be visualized in its entirety, as if a radiological contrast medium had been used. In this way, areas of high and/or altered “hematic” signal indicate the presence of the vascular malformation (Fig. 28.2).
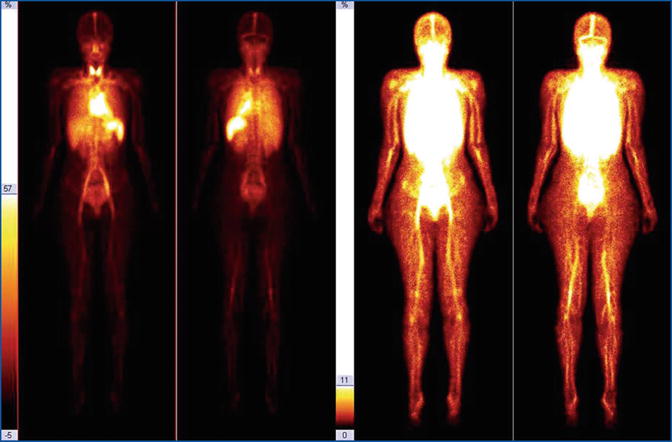
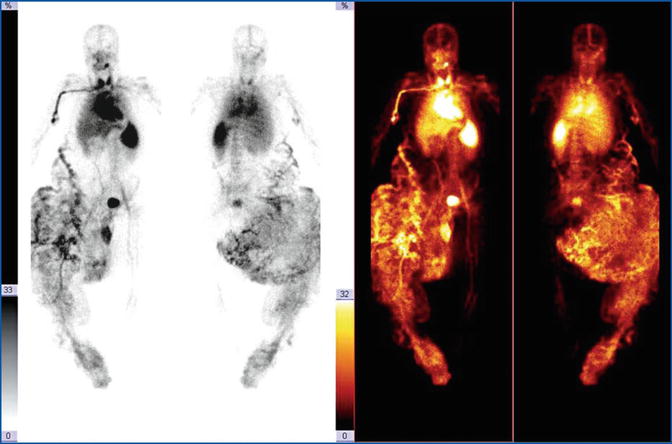

Fig. 28.1
Red blood cell angioscintigraphy: normal whole-body scan

Fig. 28.2
Young patient with right lower limb overgrowth. The labelled RBC study demonstrates a total subversion of the vascular tree of the right leg. The dysplastic mass is spreading to the abdominal wall, partially surrounding the splanchnic tissues. The tomographic three-dimensional reconstruction emphasizes the chaotic structural complexity of this malformation
As an example, in the truncular forms of vascular malformation, it is possible to distinguish anomalies of both arterial and venous course, stenotic occlusions, and expansions of the vessels. The poor or absent evidence of an artery or vein section, such as the slowing down or the absence of downstream flow, demonstrates hypoplasia or aplasia of the vessel, respectively (Fig. 28.3). Aneurysms are also recognizable with a typical and irregular increase in caliber.
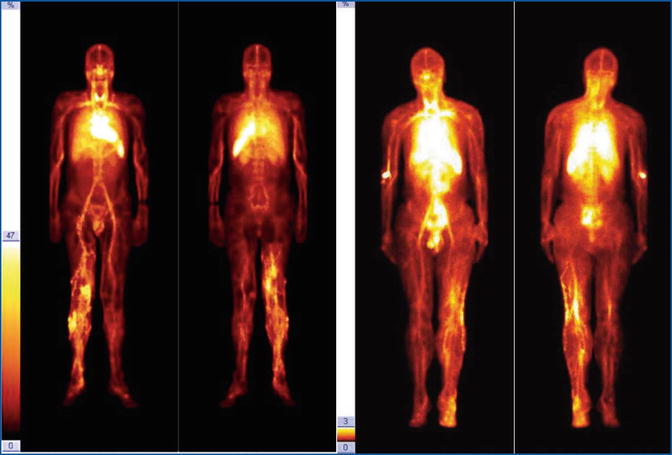

Fig. 28.3
Two cases of marginal vein of the inferior limb. Deep venous trunks are faintly visualized
In the extratruncular forms of vascular malformation, the tracer “accumulates” in pathological areas, providing a clear vision of the extent of the dysplasia. With images acquired in mutually orthogonal projections and especially with the three-dimensional tomographic reconstruction, it is also possible to better define and locate the lesion.
Of great interest is the possibility of identifying vascular malformations that, because of their liquid or mucinous aspect, can deceive sophisticated methods such as computer tomography (CT) and NMR: areas already catalogued as “angiomatous” but that are “cold” (not vascularized) according to scintigraphy should be carefully reexamined. Finally, in some cases, whole-body acquisition can reveal hidden abnormalities.
Furthermore, the study can be extended to the following day: this is possible because the biological contrast medium, represented by radiolabelled erythrocytes, remains active throughout its radioisotope decay. This is particularly suitable for the evaluation of lymphovenous abnormalities in which the slow recirculation time and mixture among the two pathological circuits do not allow an immediate evaluation using the most traditional radiological techniques, which are limited by the rapid elimination of contrast medium (Fig. 28.4).
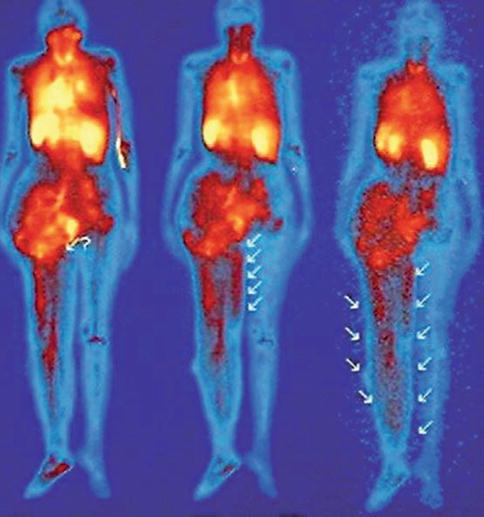

Fig. 28.4
Lymphovenous shunt, detected by angioscintigraphy. At the second-day scan, a progressive subdermal hematic spillage is observed (arrows) (Reproduced from Dentici et al. [3])
It is possible to view the “discharge” or the “effusion” of the autologous cells from the intravascular compartment to the lymphatic one, achieving results of absolute diagnostic value. The main limitation of this method is the poorly detailed image, which does not allow discrimination between venous and arterial structures.
It is possible to differentiate between high-flow malformations (arteriovenous malformations [AVM]) and low-flow masses (prevailing venous component) by the comparative analysis of the transit time of the tracer in the pathological and corresponding healthy districts: the presence of an AVM is almost always revealed by activity-time curves obtained by the acquired dynamic sequence during the intravenous administering of radiotracer. The estimation of intralesional hemodynamics is essential in determining the appropriate treatment: surgical resection, arterial embolization, or sclerotherapy (Fig. 28.5).
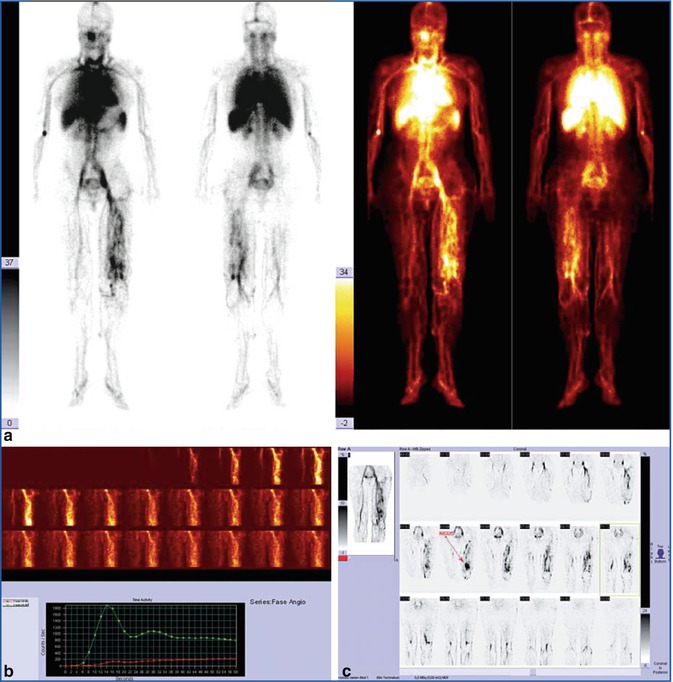

Fig. 28.5
(a) In this patient, the whole-body scan demonstrates a complex intra- and extratruncular malformation. (b) Study of the tracer’s transit time is barely suggestive of an arteriovenous shunt. (c) At tomographic reconstruction, the AVM nidus is visible above the knee joint and in front of femoral artery
Flow study (with the analysis of transit time or with direct puncture into the intravascular space of the lesion) provides a quantitative indicator of intralesional hemodynamics in low-flow lesions, in addition to accurate distinction between high-flow and low-flow lesions. For the percutaneous injection of sclerosing agents, the estimation of the flow characteristics of soft tissue vascular anomalies is essential for determining appropriate patient management. The last promising methodological aspect of great interest is represented by whole-body tomographic study, which can be extended to any corporeal district.
The tomographic approach in nuclear medicine has been used for the study of cerebral and myocardial perfusion (with ad hoc radiolabelled tracers) and for the evaluation of vascular malformations (prevalently hepatic), thanks to the possibility of revealing the “vascular nature” of lesions, not seen with methods such as echography, CT angiogram, and NMR.
The most recent generation of nuclear equipment and the availability of iterative rebuilding algorithms allow images of higher definition and total body images in three-dimensional rotation to be obtained; these can help to better define the highlighted lesions and their topographical relationships.
The availability of updated image-fusion software allows exams acquired with radiological methods to be imported and nuclear medicine imaging (NMI), CT, and NMR images to be melted, obtaining an iconography of high diagnostic content, in which structural and functional aspects of primary importance are merged in areas not accessible for clinical or sonographic exams, for instance, angiomatous intracranial or facial malformations (Fig. 28.6).
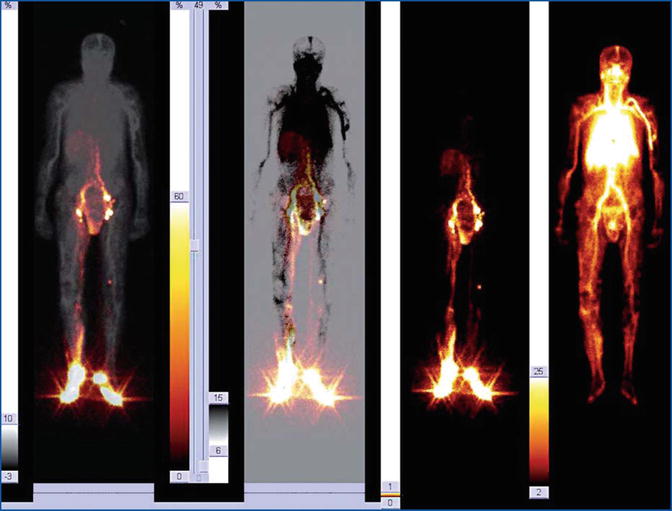

Fig. 28.6
Fusion imaging of a complex composite malformation involving the right inferior limb and the pelvis. The venous dysplasia mainly includes the calf and the lateral side of the knee and thigh. The lymphatic defect involves dermal effusion of the calf, the internal side of the thigh, the scrotum, and the peritoneal space, configuring a picture of probable lymphocele complicated by abdominal spillage. No evidence of lymphovenous shunt
Recently, WBBPS was evaluated in 137 patients suspected to have venous or lymphatic malformations as a whole-body screening and diagnostic tool. WBBPS successfully detected abnormal blood pooling lesions in 96.8 % (120/124) of the patients with venous malformations, while no tracer entered in lymphatic defects, demonstrating a good differential diagnosis possibility. In addition, WBBPS discovered 41 other lesions not recognized by clinical evaluation. WWBP was considered a valuable initial diagnostic tool with an accuracy of 97.1 % [4].
For postoperative evaluation of results of treatment, WBBPS was successfully used; a positive result after alcohol treatment was considered the reduction of the 50 % decrease in abnormal blood pool ratio compared with pretreatment images [5].
This new gold standard is today within the reach of nuclear equipment supplemented with a single “hybrid” diagnostic position, a multihead gamma camera, and a multislice CT.
The integration of NMI and NMR is anticipated in the near future.
Lymphoscintigraphy
Historically, the instrumental approach to the alterations of the lymphatic system was carried out by radiological lymphangiography with a contrast medium. This method represents the gold standard for the study of a series of alterations and compromises of the lymphatic system, from the simple lymphedema to the staging of neoplasia involving the pelvis and the abdominal area [6].
While this method is able to reveal every detail of the lymphatic grid, escape routes, the presence of morphological alterations, and the caliber of vessels, it does have some drawbacks, which relegate it as an historical instrument of the radiologist rather than as one of the active tools of diagnostic practice.
Radiological lymphangiography is technically very difficult, requiring a microsurgical approach to the interdigital lymphatic capillaries; it also employs an oily contrast medium that remains in circulation and which may cause local complications at the injection point (up to skin necrosis), may be the cause of lymphangitis (in some cases worsening the clinical picture and causing functional damage that may exacerbate lymphedema), and, in extreme situations, may cause distant oily emboli, with the passage of the contrast medium into the blood stream. Taking into consideration these quite major problems, scintigraphy provides an alternative that is able to provide high-quality information and requires no special training. In addition, scintigraphy is not invasive, harmless to the lymphatic endothelium, devoid of collateral adverse effects (and therefore well tolerated by the patient), economic, repeatable, and especially reproducible (Fig. 28.7).