Novel Immunologic Therapies
Leslie C. Grammer
Allergic diseases are very prevalent, afflicting up to 20% of the American population; novel immunologic approaches to their abatement are avidly pursued. These approaches generally can be divided into three strategies. One approach is to administer monoclonal antibodies against molecules, usually proteins, that have been reported to be key in mediating allergic inflammation. Another is to administer other monoclonal proteins that will interfere with the allergic inflammatory process. A final strategy is to modify allergen immunotherapy using innovative techniques to reduce allergenicity and maintain and/or enhance immunogenicity.
Monoclonal Antibodies
Monoclonal Anti–immunoglobulin E
The elimination of immunoglobulin E (IgE) to provide an effective therapy for allergic diseases is based on the importance of IgE in both early- and late-phase reactions (1). Various strategies have been used to interfere with the binding of IgE to its receptors, thus abrogating allergic disease. Examples include inhibiting IgE production, use of IgE fragments to occupy the receptor, administration of soluble receptors to bind free IgE, and neutralizing antibodies against IgE. Polyclonal and monoclonal anti-IgE antibodies have been produced to study mechanisms of allergic disease (1). Omalizumab is a recombinant humanized monoclonal antibody that is reported to be effective for the treatment of patients with moderate-to-severe persistent asthma who have IgE-mediated disease not controlled by corticosteroids (2–4). In addition to reducing free IgE, other mechanisms of action, including changes in eosinophil and T cell function as well as reduction of FcεRI expression on dendritic cells, mast cells, and basophils, have been described (5).
In a ragweed rhinitis trial, some symptomatic improvement was described in patients who had markedly reduced free IgE levels and markedly increased bound IgE levels (6). Trials of omalizumab for atopic dermatitis have reported both positive and negative results (7,8). To date, there have been no reports of omalizumab inducing an antibody response in humans. While the most common side effect has been the development of urticarial eruptions, patients have developed other adverse effects, including the rare possibility of anaphylaxis (9,10).
Anti–interleukin-5
Interleukin-5 (IL-5) is a helper T-cell type 2 (TH2) cytokine that is reported to be essential for the recruitment and proliferation of eosinophils in the allergic inflammatory response. In animal models, anti–IL-5 blocking antibody has been reported to inhibit eosinophil recruitment and ablate the late-phase response (11). A humanized anti–IL-5 blocking antibody (mepolizumab) is, at the time of this writing, in clinical trials for the treatment of hypereosinophilic syndrome (HES), eosinophilic esophagitis (EE), severe asthma, and nasal polyposis (12). Mepolizumab received orphan drug status for treatment of patients with HES in the United States and the European Union in 2004. It should be noted that there are subgroups of HES, individuals with FIP1L1-PDGFRA fusion gene (F/P+ variant) or increased IL-5 production by a clonally expanded T-cell population (lymphocytic variant), most frequently characterized by a CD3–CD4+ phenotype. For F/P+ patients, imatinib, a low-molecular-weight tyrosine kinase inhibitor, has become first-line therapy (13). Recent data suggest that mepolizumab is an effective corticosteroid-sparing agent for F/P-negative patients. Mepolizumab is in phase I/II clinical development for the treatment of EE. When administered intravenously to allergic asthmatic subjects, it reduced both blood and sputum eosinophilia, but did not reduce airway hyperresponsiveness or allergen-induced late-phase response. These results question the association between IL-5 and allergic disease, but do not preclude its importance in disorders that are known to be eosinophil-mediated (12).
Anti–tumor Necrosis Factor-α
It is well recognized that tumor necrosis factor-α (TNF-α) is involved in the inflammation of certain TH1-associated diseases like Crohn, psoriasis, and rheumatoid arthritis. In those diseases, anti-TNF-α therapies have produced significant clinical improvement. In patients with severe, steroid dependent asthma, TNF-α may be upregulated as well, resulting in the recruitment of neutrophils and eosinophils into the airways (14). While there was initial enthusiasm for anti-TNF-α therapy, this has been dampened by concerns over safety. Moreover the efficacy of anti-TNF-α therapy is likely to be confined to a small subgroup of patients with severe asthma. There is an increasing recognition that there is considerable phenotypic heterogeneity in severe refractory asthma. Therefore, it seems that the utility of anti-TNF-α as a novel therapy will be limited to phenotypes that are highly selected (15). In severe, recalcitrant atopic dermatitis (AD), anti-TNF therapy may be a consideration (14).
Other Monoclonal Antibodies and Fusion Proteins
There are several monoclonal antibodies, developed and approved for other diseases, that have been reported to be efficacious in allergic diseases. In general, the intensity of the allergic disease is severe, thereby justifying the known adverse effects. In a small trial of anti-CD20 (rituximab) treatment, improvement in severe AD was reported (16). Although well tolerated, only two of nine patients with moderate-to-severe AD benefited from treatment with alefacept, a fusion protein which combines part of an antibody with a protein that blocks the growth of some types of T cells (17).
Integrins are validated drug targets that might be useful in a variety of inflammatory diseases (18). Monoclonal antibodies that antagonize integrin alphaIIbbeta3 (e.g., abciximab), integrin alphaIbeta2 (efalizumab), and integrin alpha4beta1 (natalizumab) are currently U.S. Food and Drug Administration (FDA)-approved for acute coronary syndromes, psoriasis, and multiple sclerosis, respectively. However, none has been approved for indications related to asthma and other allergic diseases. Since selectins and integrins play key roles in respiratory inflammation, it is possible that monoclonal antibodies against them may be of use in asthma and other allergic diseases (18).
Another important target for intervening in allergic disease emerged with the discovery that chemokines belonging to the CC family (which includes RANTES and MCP-1, -3, and -4) are potent eosinophil chemoattractants that use a common receptor, CCR3. A monoclonal antibody to CCR3 has been reported to cause inhibition of eosinophil migration (19). Using bacteriophage expression libraries and combinatorial chemistry, highly selective CCR3 antagonists have been discovered. Low-molecular-weight approaches have supplanted monoclonal antibodies; CCR3 antagonists have been associated with decreases in tissue eosinophilia and airway hyperreactivity in animal models (20,21).
Other Monoclonal Proteins
Soluble Interleukin-4 Receptor
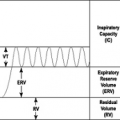
Interleukin-4 (IL-4) plays an important proinflammatory role in asthma through several mechanisms, including stimulation of TH2 lymphocytes, which results in production of IL-5, IL-13, and more IL-4. When IL-4 is absent, TH2 lymphocyte differentiation is inhibited. Several studies have reported the results of using soluble IL-4 receptor (sIL-4R) as a treatment for allergic disease. In one such randomized, placebo-controlled trial of 25 moderate asthmatics, all requiring inhaled corticosteroids, there was improvement in forced expiratory volume in 1 second (FEV1) on the fourth day of aerosolized sIL-4R treatment in the high-dose group (22). There were no serious side effects of sIL-4R. In another study, sIL-4R was evaluated in steroid dependent asthma patients following the withdrawal of inhaled corticosteroid (ICS) therapy (23). Anti-inflammatory activity was shown by a reduction in exhaled nitric oxide after a single sIL-4R dose; stabilization of asthma symptoms also occurred, despite ICS therapy withdrawal. When administered once weekly for 12 weeks, sIL-4R stabilized the FEV1. However, the study discontinuation rate due to asthma exacerbation was similar between the sIL-4R and placebo groups. No recent clinical evaluation has been reported.
Interferons
Recombinant interferon-γ (IFN-γ) is available as a therapy approved by the FDA for chronic granulomatous disease. IFN-γ is known to suppress IgE production and to downregulate the function and proliferation of CD4+ TH2 cells (24). The role of interferons in IgE-mediated diseases probably will be restricted to very severely affected patients because the risk for side effects, including fever, chills, headache, rash, depression, and even suicide, generally outweigh any possible benefit (25). Clinical improvement has been reported in patients with severe atopic dermatitis (26).
Modified Allergen Immunotherapy
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree