Non-Small Cell Lung Cancer
INTRODUCTION
 EPIDEMIOLOGY
EPIDEMIOLOGY
Lung cancer is the leading cause of cancer-related mortality in the United States, with an estimated 160,000 deaths annually. Although lung cancer deaths have been declining among men since the early 1990s, lung cancer mortality has only recently begun to decline in women, likely reflecting gender differences in cigarette smoking and tobacco cessation patterns over the last 50 years. Cigarette smoking is the strongest modifiable risk factor for the development of lung cancer, accounting for 85%–90% of cases (1). Furthermore, smokers have a 20-fold increased risk of death from lung cancer compared to non-smokers. Still, other risk factors exist, including asbestos exposure, ionizing radiation, and exposure to carcinogenic chemicals and minerals. Research is ongoing regarding dietary and genetic risk factors.
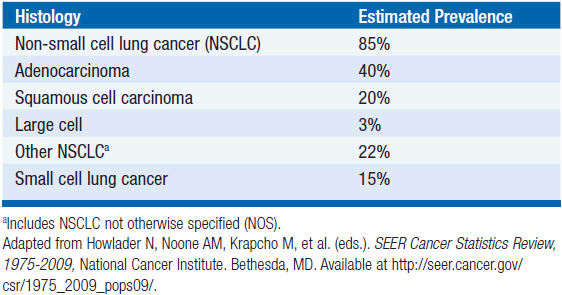
Lung cancer is traditionally divided into two major classes: small cell lung cancer and non-small cell lung cancer (NSCLC). NSCLC accounts for approximately 85% of lung cancer cases. NSCLC can be further classified based upon histopathologic designations that include adenocarcinoma, squamous cell carcinoma (SqCC), and large cell carcinoma. Historically, SqCC was the most frequent type of NSCLC; however, adenocarcinoma has become twice as common as SqCC in the last 40 years, perhaps reflecting changes in cigarette composition over this time period (Table 51-1). Recently, the classification of lung adenocarcinoma was revised to provide more uniform terminology and diagnostic criteria across multidisciplinary providers (2). Notably, this revised classification scheme eliminated the category of bronchioloalveolar cell carcinoma (BAC).
 PRESENTATION
PRESENTATION
The majority of patients with NSCLC are symptomatic at diagnosis. The most common symptoms arising from the primary tumor are cough, dyspnea, blood-tinged sputum, and chest pain. Local extension of the tumor within the chest can cause pleural or pericardial effusions, chest pain, hoarseness, brachial plexopathy, Horner’s syndrome, and superior vena cava syndrome. Metastatic disease may present with weight loss, neurologic symptoms, or bony pain. Paraneoplastic syndromes such as syndrome of inappropriate antidiuretic hormone secretion (SIADH), Cushing’s syndrome (ectopic corticotropin secretion), and Lambert-Eaton myasthenic syndrome are more commonly associated with small cell lung cancer, but NSCLC may be associated with hypercalcemia of malignancy or hypertrophic pulmonary osteoarthropathy.
 SCREENING
SCREENING
Given the global burden of lung cancer deaths, a number of different screening strategies have been explored in order to improve lung cancer detection and survival. In the last three decades, multiple randomized trials using screening chest radiographs with or without sputum cytology failed to show reductions in lung cancer mortality (3–5). More recently, several small trials have suggested that low-dose helical computed tomography (CT) detected more nodules and early-stage lung cancers compared to chest radiography. These results prompted several large randomized screening trials using low-dose CTs, including the National Lung Cancer Screening Trial (NLST) and the ongoing Dutch-Belgian randomized lung cancer screening trial (NELSON). In 2011, results from the NLST were published, demonstrating a 20% relative risk reduction in lung cancer mortality among high-risk patients undergoing annual screening with low-dose CT compared to chest radiographs (6). High-risk patients in the NLST were between 55 and 74 years of age, were current or former smokers (quit ≤15 years), had at least a 30 pack-year smoking history, and had no prior history of lung cancer. Although several organizations have since recommended lung cancer screening using low-dose helical CTs based upon these data, the U.S. Preventative Services Task Force has not endorsed screening to date.
 STAGING AND PROGNOSIS
STAGING AND PROGNOSIS
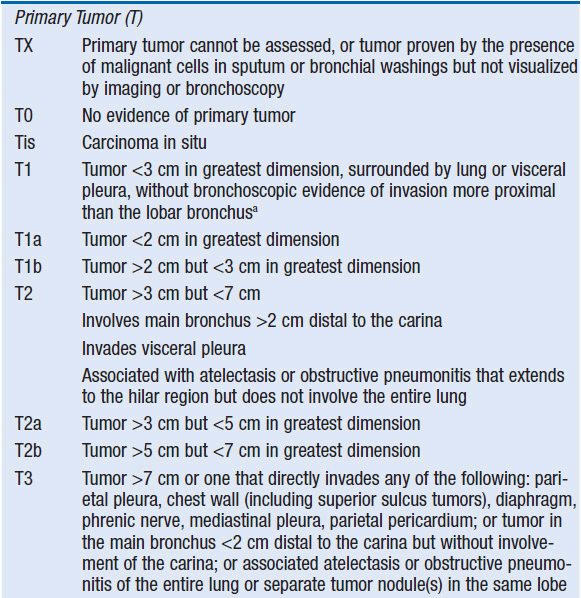
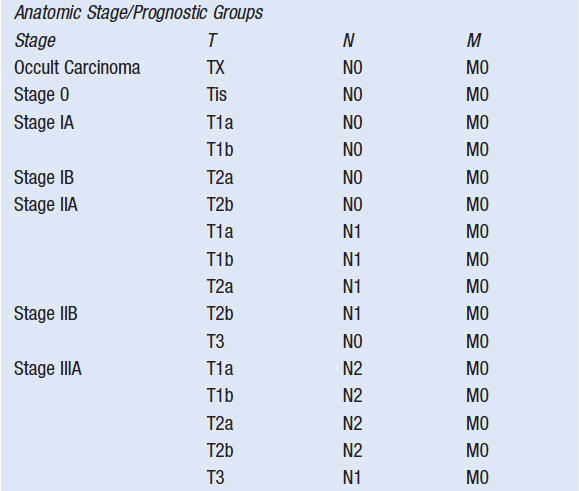
The primary determinant of prognosis in NSCLC is stage at diagnosis. Stage is defined by the size of the primary tumor and involvement of regional lymph nodes and metastatic sites via the American Joint Committee on Cancer TNM system (Table 51-2). Significant revisions to this system were implemented in 2009.
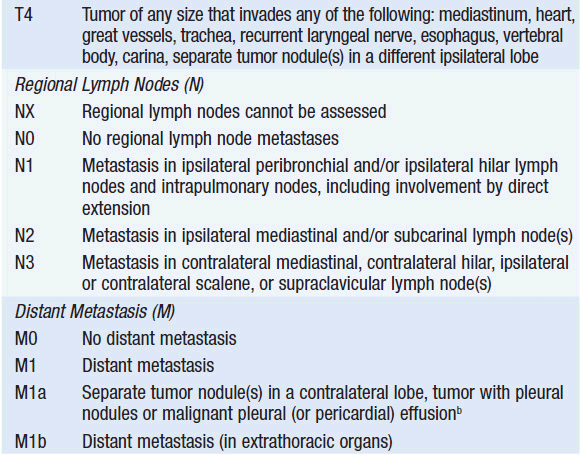
All NSCLC patients should undergo a contrast-enhanced chest CT scan extending through the liver and adrenal glands, as well as a whole body PET scan to identify occult metastases. MRI of the brain is also encouraged. In the absence of metastatic disease, the nodal (N) status is the most important determinant of overall stage and therefore of prognosis. Thus, management decisions often depend on N staging. Nodal involvement may be assessed by clinical means such as CT and PET scan, or more accurately by pathologic evaluation via biopsy. CT scans have a sensitivity of 61% and a specificity of 79% for detection of involved mediastinal nodes in NSCLC, while PET scan is slightly better with a sensitivity of 85% and a specificity of 90% (7). Nevertheless, in the absence of obvious metastatic disease, pathologic assessment of mediastinal lymph nodes via mediastinoscopy is a vital component of NSCLC staging. Recently, alternative approaches to mediastinal lymph node sampling have been advanced, such as endobronchial ultrasound and esophageal ultrasound (8).
EARLY STAGE NSCLC
 SURGERY
SURGERY
Surgical resection of the tumor and draining lymph nodes is the cornerstone of therapy for stages I–II NSCLC and provides the greatest likelihood of cure. The type of surgery performed depends on tumor size, location, overall health of the patient, and local practice standards. Pulmonary resections are classified as anatomic if they encompass the draining lymphovascular structures, as in pneumonectomy, lobectomy, and segmentectomy, or non-anatomic, as in wedge resection. Resections can be performed through a standard thoracotomy incision, or in some cases through a smaller incision with video thoracoscopic assistance. Complete resection with a clean margin is the strongest predictor of cure after NSCLC surgery; hence, the location and size of the tumor partially dictate the required operation.
An additional consideration is the amount of lung parenchyma to be resected and the resultant morbidity and mortality. A randomized study of peripheral stage IA NSCLC tumors treated with lobectomy versus a limited surgery (segmentectomy or wedge) found that limited surgery was associated with an increased risk of local recurrence and a trend toward inferior survival. The methodology of this study has been criticized due to its statistical design (e.g., lack of intention to treat in primary analysis), lack of modern staging techniques (e.g., CT, PET), and limited long-term, postoperative pulmonary function reporting (9). Nonetheless, lobectomy is the preferred operation for NSCLC unless tumor characteristics or patient comorbidities dictate otherwise. Hospital surgical volume also correlates inversely with perioperative mortality.
 MEDICALLY INOPERABLE PATIENTS
MEDICALLY INOPERABLE PATIENTS
Patients with early-stage NSCLC and excessive surgical risk due to medical comorbidities are termed “medically inoperable.” Characteristics such as current smoking, poor exercise capacity, weight loss, and severe COPD are associated with high-surgical risk. Advanced age alone should not preclude an otherwise fit patient from surgery as retrospective analyses suggest survival among elderly patients is similar to younger patients after resection of early stage NSCLC (10, 11).
Options for medically inoperable patients include standard radiation therapy (RT), stereotactic body radiotherapy (SBRT), radiofrequency ablation (RFA), or observation. Standard RT yields about one-third to one-half the cure rate achieved surgically, with a 5-year OS of 20%–30% for stage I NSCLC (12). More recently, the introduction of SBRT, also known as stereo-tactic ablative radiotherapy (SABR), has changed the approach to medically inoperable patients with early-stage NSCLC. SBRT involves the delivery of precise, high-dose radiation over a single or limited number of dose fractions. In observational studies of SBRT, medically inoperable patients with early-stage NSCLC experienced local tumor control rates of greater than 90% and a 3-year OS of nearly 60% following treatment with SBRT (13). No randomized trials have been performed comparing SBRT and standard RT. However, based upon historical comparisons of local tumor control rates, SBRT is generally considered standard of care for patients with early-stage NSCLC who are deemed medically inoperable or who decline surgical resection. Studies comparing SBRT versus sublobar surgical resection in high-risk patients (i.e., able to tolerate sublobar resection but not lobectomy) are ongoing.
 ADJUVANT TREATMENT
ADJUVANT TREATMENT
Despite surgery, 50%–60% of patients with early-stage NSCLC will relapse and die from their lung cancer. Postoperative, or adjuvant, chemotherapy and RT aim to decrease the risk of local and distant recurrence. Adjuvant RT can decrease local recurrence rates but does not impact overall survival. A large meta-analysis even suggested adjuvant RT could increase the risk of death for stage I and II patients (14). In general, adjuvant RT is not standard of care treatment, but it may be considered for specific patients with positive or close surgical margins or with incidentally discovered stage III disease at the time of resection.
Distant metastases are the most common source of treatment failure following potentially curative surgery in early-stage NSCLC, providing a rationale for adjuvant chemotherapy. In 1995, a large meta-analysis suggested that adjuvant cisplatin-based chemotherapy conferred a survival benefit in patients undergoing resection for NSCLC, although this difference was not statistically significant (15). Subsequently, multiple randomized trials have demonstrated a 3%–15% improvement in survival with cisplatin-based adjuvant chemotherapy. In 2008, data from five of these trials were combined in the Lung Adjuvant Cisplatin Evaluation (LACE) meta-analysis (16). In this pooled analysis of 4584 patients with over 5 years of follow-up, adjuvant cisplatin-based chemotherapy was associated with an absolute survival benefit of 5.4% at 5 years. This benefit was most pronounced in patients with stage II and III disease and those with favorable performance status. In these patient populations, cisplatin-based adjuvant chemotherapy is therefore considered standard of care. Based upon subgroup analyses from available randomized trials, some patients with stage IB NSCLC (e.g., tumor size >4 cm) may also benefit from adjuvant chemotherapy.
 STAGE III NSCLC
STAGE III NSCLC
Stage III NSCLC, or locally advanced disease, encompasses a heterogeneous group of tumors. The vast majority of patients with stage IIIA disease are so designated because of N2 lymphadenopathy, meaning involvement of the ipsilateral mediastinal or subcarinal nodes (Figure 51-1). In general, treatment of stage IIIA NSCLC requires multiple therapeutic modalities. Based upon patient and tumor characteristics, treatment may consist of induction chemotherapy followed by surgery, concurrent chemoradio-therapy followed by surgery, or definitive chemoradiation without surgery. Unfortunately, clinical trials comparing such approaches have been limited by patient heterogeneity, poor accrual, and imprecise staging. Thus, the optimal management approach is still controversial, particularly with respect to induction strategies. Indeed, in a 2010 survey of practice patterns for stage IIIA NSCLC among the National Comprehensive Cancer Network (NCCN) member institutions, 50% of institutions reported use of induction chemotherapy as the predominant treatment approach while 50% reported using neoadjuvant chemoradiotherapy in a majority of cases (17).
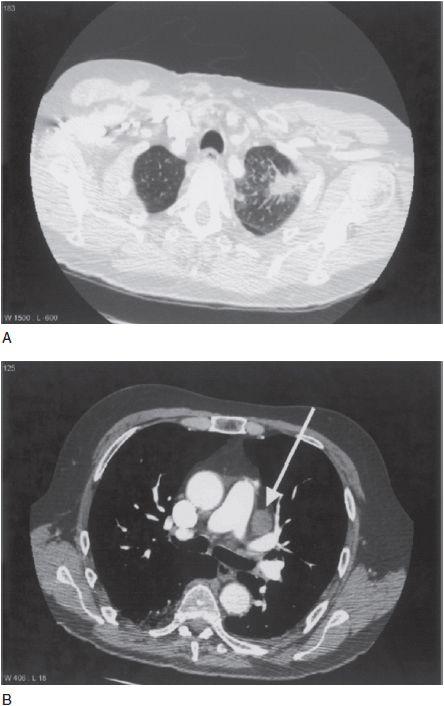
FIGURE 51-1 Clinical stage IIIA NSCLC. Panel A shows a spiculated left upper lobe primary tumor. Panel B shows bulky ipsilateral mediastinal lymphadenopathy (arrow).
Another area of controversy in the management of stage IIIA disease is the role of surgical resection following induction therapy. As noted above, treatment approaches for stage IIIA tumors include definitive concurrent chemoradiation or induction therapy (e.g., neoadjuvant chemotherapy or neoadjuvant chemoradiation) followed by surgical resection. The U.S. Intergroup 0139 trial was designed to assess the role of surgical resection as part of these strategies (18). This trial randomized 429 patients with stage IIIA NSCLC and positive N2 nodes to definitive cisplatin-based chemoradiotherapy or neoadjuvant chemoradiotherapy followed by surgery. An interim analysis showed no difference in survival between the two arms. However, the definitive chemoradiotherapy arm had a higher rate of local recurrence, while the surgical arm had more early deaths due to perioperative complications, especially among patients requiring pneumonectomy. In fact, a subgroup analysis of the patients undergoing lobectomy and matched nonsurgical controls showed an increased median survival in the lobectomy group (34 months) compared to the definitive chemoradiotherapy group (22 months, p = 0.002). The interpretation of these data has been that tumors amenable to lobectomy likely benefit from neoadjuvant therapy, but tumors requiring pneumonectomy should be treated by definitive chemoradiotherapy without surgery. If there is progressive disease or clinical decline during neoadjuvant treatment, the prognosis is poor and resection should no longer be considered.
Locally advanced NSCLC also encompasses stage IIIB disease. Stage IIIB tumors are by definition unresectable, either due to involvement of vital structures or due to spread to the contralateral lymphatic system. In general, the standard of care for patients with stage IIIB disease and a good performance status is definitive, concurrent chemoradiation. This is based upon randomized trial data demonstrating that cisplatin-based chemotherapy given prior to RT results in improved survival compared to RT alone (5-year OS 17% vs. 6%, respectively) (19, 20). Furthermore, cisplatin-based chemotherapy given concurrently with RT is superior to sequential chemotherapy and RT (5-year OS 15.8% vs. 8.9%, respectively) (19, 21). In the United States, the most commonly used chemotherapy regimens in this setting are: (1) cisplatin and etoposide, and (2) weekly carboplatin and paclitaxel. Currently, the combination of cisplatin and pemetrexed with concurrent radiation is also being evaluated in ongoing clinical trials for patients with stage IIIB nonsquamous NSCLCs.
 PANCOAST TUMORS
PANCOAST TUMORS
Approximately 5% of lung cancers are located in the superior sulcus and are termed pancoast tumors. This designation encompasses tumors of various stages, but they share distinct presenting signs and symptoms, such as arm pain, numbness, and weakness from involvement of the brachial plexus. Limited space in the superior sulcus makes immediate surgical resection technically difficult. As a result, pancoast tumors were historically treated with neoadjuvant RT followed by surgery, although this approach yielded 5-year OS rates of only 30%. The success of neoadjuvant chemoradiotherapy in stage III NSCLC led to a single-arm, phase II trial of this approach in patients with pancoast tumors with T3-4 and N0-1 status (22). A total of 110 patients received neoadjuvant chemoradiotherapy followed by surgery. Five-year OS was 44%, an improvement compared to historical findings with RT and surgery. Interestingly, pathologic complete and near-complete responses exceeded radiographic complete response rates, suggesting that there are often residual abnormalities on CT scan that do not contain viable tumor tissue.
ADVANCED NSCLC
Approximately 40% of patients with NSCLC present with advanced or stage IV disease. Advanced NSCLC is an incurable condition, and the goals of treatment are both to extend life and to palliate. In general, treatment decisions for advanced NSCLC are based upon a number of factors, including tumor histology, patient performance status (PS), and the patient’s overall medical condition. Additionally, in the last decade, management approaches for NSCLC have undergone a significant paradigm shift, with an emphasis on stratifying patients based upon the molecular characteristics of their tumors. Such strategies have been informed by the recent success of targeted therapies in patients with genetic alterations in the EGFR and anaplastic lymphoma kinase (ALK). We will begin this section with a discussion of standard first-line chemotherapy in advanced NSCLC, after which we will address these emerging molecular targets (see “Targeted Therapy for NSCLC”).
 FIRST-LINE CHEMOTHERAPY FOR ADVANCED NSCLC
FIRST-LINE CHEMOTHERAPY FOR ADVANCED NSCLC
Chemotherapy is a mainstay of treatment for patients with advanced NSCLC. Early evidence of the benefits of chemotherapy in advanced disease came in the form of a 1995 meta-analysis of 11 trials, which demonstrated an approximate 2-month improvement in overall survival with platinum-based chemotherapy compared to best supportive care (15). Several additional studies have since confirmed this survival advantage and shown that chemotherapy improves quality of life for patients with advanced NSCLC and a good performance status (ECOG PS 0 or 1).
In general, standard management for advanced NSCLC consists of two-drug platinum-based combination regimens (doublets). Cisplatin-based regimens appear to offer a slight OS benefit compared to carboplatin-based regimens (23); however, this difference is clinically significant only in the treatment of early-stage disease, when cure is possible. Thus, in the United States, most clinicians prefer to use carboplatin for advanced disease because it has a more favorable side-effect profile.
A number of clinical trials have attempted to identify the optimal doublet for advanced NSCLC. In 2002, results from a phase III randomized trial comparing four different platinum-based doublets were published (24). In this study of 1155 patients with advanced NSCLC, no differences in overall survival were observed among those receiving cisplatin/paclitaxel, cisplatin/gemcitabine, cisplatin/docetaxel, or carboplatin/paclitaxel. Each doublet produced response rates of nearly 20% and a median survival of approximately 8 months.
Until recently, clinicians choose between platinum-based doublets based upon local practice patterns and patient preferences regarding side-effect profiles and schedules of administration. However, several randomized phase III trials have since highlighted the importance of tumor histology as an important determinant in treatment decisions. In a recent phase III non-inferiority trial comparing cisplatin/gemcitabine versus cisplatin/pemetrexed in 1725 previously untreated patients, there was no difference in median OS between the two arms (25). In preplanned subgroup analysis, however, patients with adenocarcinoma histology treated with cisplatin/pemetrexed had a significant improvement in median OS compared to those treated with cisplatin/gemcitabine (12.6 vs. 10.9 months). Conversely, in patients with SqCC histology, cisplatin/gemcitabine produced superior survival compared to cisplatin/pemetrexed (median OS 10.8 vs. 9.4 months). Thus, pemetrexed is now approved as part of first-line treatment in advanced, nonsquamous NSCLC.
In recent years, multiple randomized trials have tried to improve upon the results of platinum-based doublet chemotherapy. Initial attempts to add a third chemotherapeutic agent resulted in increased toxicity without a benefit in survival. More recently, efforts have included adding monoclonal antibodies. Bevacizumab is a monoclonal antibody that targets vascular endothelial growth factor (VEGF). In 2006, results from the landmark ECOG 4599 trial were published, demonstrating an OS benefit with the addition of bevacizumab to carboplatin/paclitaxel chemotherapy (26). Median survival was 10.3 months in the chemotherapy arm and 12.3 months in the chemotherapy plus bevacizumab arm. The population eligible for ECOG 4599 was relatively selective, excluding patients with brain metastases, those with SqCC histology, and those with significant risks of bleeding or thrombosis. Nevertheless, ECOG 4599 represents a major advance in the primary treatment of NSCLC as it is the first randomized trial to show a median survival of greater than 1 year. The triplet regimen with bevacizumab is now considered standard of care treatment for patients who match the study eligibility criteria.
 MAINTENANCE THERAPY
MAINTENANCE THERAPY
First-line chemotherapy should be discontinued at the time of disease progression. For patients who respond or have disease stabilization with chemotherapy, continuation of a platinum-based doublet beyond 4–6 cycles does not appear to improve OS. However, there has recently been renewed interest in the notion of maintenance therapy, which aims to prolong the duration of disease control (either response or stable disease) following completion of a predefined number of induction chemotherapy cycles. Two different maintenance strategies have emerged. Continuation maintenance describes treatment with at least one of the initial agents from the induction regimen, typically the non-platinum compound. Switch maintenance refers to the introduction of a new drug that was not used as part of the induction regimen.
Various agents have been evaluated in the maintenance setting, including multiple non-platinum cytotoxic agents as well as molecularly targeted therapies, such as VEGF and EGFR inhibitors. In recent randomized phase III trials, both gemcitabine and pemetrexed have demonstrated improvements in progression-free survival (PFS) when administered as continuation maintenance, although only pemetrexed has conferred an OS benefit (27). Bevacizumab is often commonly administered as continuation maintenance therapy as well. This is based upon the study design of ECOG 4599 (see “First-Line Chemotherapy for Advanced NSCLC”); however, no prospective comparisons of maintenance bevacizumab versus placebo have been performed. Phase III trials evaluating the combination of maintenance pemetrexed with or without bevacizumab are ongoing.
In randomized trials evaluating switch maintenance, several agents have produced improvements in PFS; however, only pemetrexed and the EGFR inhibitor erlotinib have produced significant improvements in OS (28, 29), Comparisons among these trials are limited by differences in induction chemotherapy regimens, patient eligibility, tumor histology, trial endpoints, and recommendations regarding treatment regimens at the time of progression. Ultimately, selection of appropriate maintenance therapy depends on a number of factors, including patient performance status, quality of life, the molecular characteristics of the tumor, response to induction chemotherapy, and tolerability of therapy.
 TARGETED THERAPY FOR NSCLC
TARGETED THERAPY FOR NSCLC
In recent years, significant advances have been made in understanding NSCLC at a molecular level. It is now recognized that “driver” oncogenes, the function of which are central role to the growth and viability of cancer cells, can be readily identified in approximately half of lung adenocarcinomas (30). Cancers harboring such mutations are often dependent or “addicted” to the corresponding oncogenic pathways for survival, providing the rationale for using therapies designed to inhibit these signaling pathways. In NSCLC, two archetypal examples of molecular targets are EGFR and ALK. As will be discussed more fully below, the success of targeted therapies in patients with genetic alterations in EGFR and ALK has resulted in a significant paradigm shift in the management of NSCLC, placing emphasis on tumor genotyping in routine clinical practice.
Gefitinib and erlotinib are oral small molecule tyrosine kinase inhibitors (TKIs) that target EGFR. In trials of these agents in the second- and third-line setting, both were associated with response rates of approximately 10% in genetically unselected patient populations. In 2004, however, somatic mutations in EGFR were discovered to predict responsiveness to EGFR TKIs (31, 32). Subsequent screening efforts identified EGFR mutations in approximately 10%–30% of NSCLC patients, with lower frequencies in Caucasian patients and higher frequencies in patients of East Asian descent. These mutations are associated with an improved prognosis as well as a number of clinical and pathologic features, including lack of smoking history, East Asian ethnicity, female gender, and adenocarcinoma histology.
In the first-line setting, treatment with gefitinib or erlotinib is associated with response rates of approximately 75% in patients with EGFR mutations. Furthermore, randomized trials have demonstrated improvements in PFS, tolerability, and quality of life in EGFR-positive patients treated with EGFR TKIs compared to conventional first-line chemotherapy. One such study was the Iressa Pan-Asia Study (IPASS), which randomized 1217 patients with advanced NSCLC to first-line treatment with either gefitinib or carboplatin/paclitaxel (33). The study population was composed of East Asian patients with pulmonary adenocarcinoma who were never or former smokers, characteristics typically associated with sensitivity to EGFR TKIs. In the overall study population, treatment with gefitinib significantly prolonged PFS compared to chemotherapy. Similarly, in the subset of patients with EGFR mutations, gefitinib resulted in an improved PFS compared to chemotherapy (9.5 vs. 6.3 months). However, among EGFR wild-type patients, gefitinib produced a significantly shorter PFS compared to chemotherapy (1.5 vs. 6.5 months), underscoring the importance of EGFR mutations, not clinical characteristics, in predicting benefit to gefitinib. Thus, tumor genotyping for EGFR mutations is now considered standard of care in advanced NSCLC.
Like EGFR mutations, chromosomal rearrangements involving the ALK gene define a distinct molecular subset of NSCLC first identified in 2007 (34). ALK rearrangements are found in approximately 4%–6% of patients with NSCLC. These alterations are essentially mutually exclusive with mutations in other oncogenic drivers, such as EGFR and KRAS. ALK translocations are also associated with unique clinical and pathologic features, including younger age, adenocarcinoma histology, and lack of smoking history. Importantly, ALK rearrangements confer marked sensitivity to the ALK TKI crizotinib. In early clinical trials in patients with ALK rearrangements, crizotinib was associated with response rates of 60% and a median PFS of 8–10 months (35). Based upon these high rates of response, crizotinib received accelerated approval by the U.S. Food and Drug Administration (FDA) in August 2011. Randomized clinical trials comparing crizotinib and conventional chemotherapy in the first- and second-line settings are ongoing, but crizotinib has already emerged as the standard of care for patients with advanced NSCLC harboring ALK rearrangements. Recently, crizotinib has also shown activity in a separate molecular cohort of patients harboring rearrangements in ROS1, which is present in approximately 1% of NSCLC (36).
The recent success of EGFR and ALK TKIs in NSCLC has validated the paradigm of stratifying and treating patients based upon molecular characteristics. Efforts are underway to find additional oncogenic driver mutations as well as identify novel therapies for existing targets (Figure 51-2).
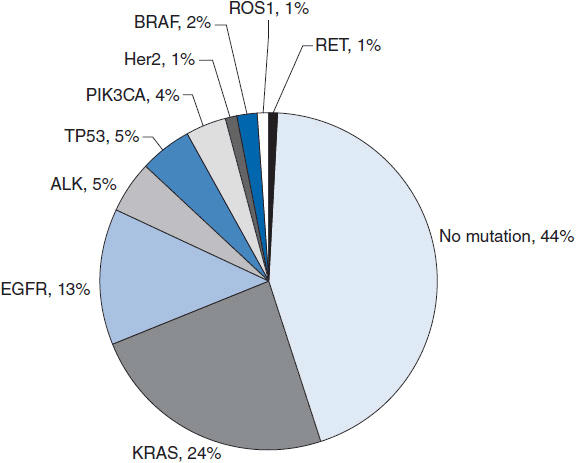
FIGURE 51-2 Distribution of driver mutations identified in lung adenocarcinomas to date.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree