21621723
Non-Hodgkin Lymphoma and Hodgkin Lymphoma
Colette Owens and Paul A. Hamlin
INTRODUCTION
Lymphomas are a heterogeneous group of malignancies of B, T, and NK lymphocytes that comprise more than 60 complex entities. By the year 2030, the United States is expected to experience an increased incidence of non-Hodgkin lymphoma (NHL) by 67% and Hodgkin lymphoma (HL) by 70% in older adults as a reflection of the aging population. Sixty percent of patients with new diagnoses of NHL are over age 65, with a median of 66 years (1). Given these demographic dynamics, the oncologist is increasingly required to manage complex older lymphoma patients in the context of age-related organ dysfunction and comorbidity.
STAGING AND INITIAL WORKUP
The updated World Health Organization schema classifies lymphomas based on morphologic, immunologic, genetic, and clinical information (2). The diagnosis of lymphoma requires adequate material for histologic, immunohistochemical (IHC), molecular, and cytogenetic analysis, preferably by an excisional biopsy, although a core biopsy is at times sufficient. Additionally, each patient should receive a careful history and physical, with blood work that includes a complete blood count, comprehensive panel, calcium, lactate dehydrogenase (LDH), uric acid, serum electrophoresis, beta-2 microglobulin, and viral testing for hepatitis B and C and HIV, and PET and/or CT chest, abdomen and pelvis. The updated Lugano staging classification of 2014 included the integration of PET scan into the initial staging of flourodeoxyglucose (FDG) avid lymphomas with the use of the Deauville 5-point scale in response assessments, eliminated the A/B designations except for HL, and eliminated routine bone marrow biopsy in diffuse large B cell lymphoma (DLBCL) and HL because of the predictive value of PET scans (3). Those at high risk for central nervous system (CNS) involvement should have a diagnostic lumbar puncture for cytology and flow cytometry.
Clinical prognostic scores are important in understanding prognoses, and help to frame treatment decisions in the older patient. Generally, there is a risk model for each major disease histology, including the International Prognostic Index 218(IPI) and Age adjusted International Prognostic Index (aaIPI), Follicular Lymphoma International Prognostic Index (FLIPI) 1 and 2, Mantle cell International Prognostic Index (MIPI), and International Prognostic Score (IPS) (Table 23.1). Age figures prominently in all of these models, with recent analysis suggesting that a more relevant inflection point for worse outcomes among elderly patients is between 70 and 75, rather than 60 years as in earlier reports (4–6,57) (Table 23.2).
TABLE 23.1 Prognostic Models Within Specific Disease Types
Disease Type | Clinical Factors | Survival Estimates |
DLBCL IPI aaIPI | Age >60 Stage III/IV PS >1 LDH > ULN ENS >2 LDH PS >1 Stage III/IV | Low (0–1) 2 y OS 84%, 5 y OS 73% Low-Int (2) 2 y OS, 66% 5 y OS 51% High-Int (3) 2 y OS, 54% 5 y OS 43% High (4–5) 2 y OS, 34% 5 y OS 26% Low (0) 5 y OS 83%, Low-Int (1) 5 y OS 69%, High-Int (2) 5 y OS 46%, High (3) 5 y OS 32% |
FL FLIPI1 FLIPI2 | Age >60 Stage III/IV Hgb <12g/L LDH > ULN >4 nodal sites Age >60 Hgb <12g/L BM involvement B2M > ULN LN diameter > 6 cm | Low (0–1) 5 y OS 91%, 10 y 71% Int (1–2) 5 y OS 78%, 10 y OS 51% High ≥ 3, 5 y OS 53%, 10 y 36% Low (0) 3 y PFS 91%, 5 y 80% Int (1–2) 3 y PFS 69%, 5 y PFS 51% High (3–5), 3 y PFS 51%, 5 y 19% |
Mantle cell lymphoma MIPI | Age Ki67 PS LDH compared ULN WBC | Low 5 y OS 81% Int 5 y OS 63% High 5 y OS 35% |
Hodgkin lymphoma IPS | Age >45 Stage IV Hgb <10.5 g/L Albumin <4 g/dL WBC >15 ALC <600 or less than 8% of total WBC count Male gender | 0 factors 5 y FFP 84%, 5 y OS 89% 1 factor 5 y FFP 77%, 5 y OS 90% 2 factors 5 y FFP 67%, 5 y OS 81% 3 factors 5 y FFP 60%, 5 y OS 78% 4 factors 5 y FFP 51%, 5 y OS 61% ≥5 factors 5 y FFP 42%, 5 y OS 56% |
ALC, absolute lymphocyte count; B2M, beta-2 microglobulin; DLBCL, diffuse large B cell lymphoma; ENS, extra-nodal sites; FFP, freedom from progression; FL, follicular lymphoma; LN, lymph node; OS, overall survival; PFS, progression-free survival; PS, performance status; ULN, upper limit of normal; WBC, white blood cell.
219TABLE 23.2 Representative Therapeutic Options in DLBCL for Older Patients
HISTOLOGIC AND BIOLOGIC RISK FACTORS
Lymphomas can be classified into indolent, aggressive, and very aggressive based on histology and outcomes. Generally, the indolent behaving lymphomas (small lymphocytic lymphoma [SLL], follicular center cell lymphoma [FCL], marginal zone lymphoma [MZL], and Waldenstrom’s macroglobulinemia [WM]) now have median survivals of more than a decade, whereas aggressive lymphomas (DLBCL, peripheral T cell lymphoma [PTCL], mantle cell lymphoma [MCL]) are life threatening within months without treatment, and highly aggressive entities (Burkitt lymphoma [BL], high-grade DLBCL) may present with rapid courses that become life threatening within weeks. Beyond these broad clinical generalizations, an increasingly nuanced understanding of biologic factors that influence outcomes is essential.
In DLBCL, the cell of origin by gene expression profiling (GEP) is prognostic for survival, with germinal center (GCB) phenotype having a better outcome than non-GC/activated B cell (ABC) phenotype with CHOP and rituximab cyclophosphamide doxorubicin oncovin (vincristine) prednisone (RCHOP) therapy (7–9). IHC models designed to approximate GEP exist (e.g., the Hans model) and are implemented given their clinical utility and availability, but problems exist with sensitivity and specificity. Ultimately, newer molecular techniques will likely supersede IHC. Additionally, the concurrent presence of immunoglobulin heavy chain translocations with the anti-apoptotic t(14;18) BCL2 and t(8;14) cMYC cellular proliferation signal (or less frequently 220BCL6 translocations) have been termed a “double hit biology” (DH). Approximately 10% of patients with DLBCL will have DH biology, which is associated with very aggressive disease behavior, older age at diagnosis, and higher CNS risk. Approximately one-third of DLBCL patients may have protein overexpression of BCL2 or BCL6 and MYC, termed “double expressers,” identified with more aggressive behavior as well, but to a more variable degree. In addition, the Epstein–Barr virus (EBV) has also been connected to the inferior outcomes in both elderly DLBCL and HL patients. These patients typically have more advanced disease, involvement of multiple extranodal sites, high risk scores, and inferior progression-free and overall survival (OS).
TREATMENT OF OLDER PATIENTS WITH LYMPHOMA
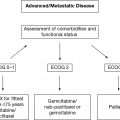
For lymphoma, the difficulties associated with clinical decision making in older patients are often most evident in the setting of aggressive histologies, such as DLBCL, MCL, and HL; hence, we will focus on these entities. Untreated, these entities will become life limiting. The goal of the medical oncologist is balancing the pursuit of curative intent in DLBCL and HL, or improvement in PFS in MCL, with the risk of morbidity and mortality in older patients. A consideration of actual life expectancy helps contextualize what treatment will mean for the patient, particularly if not treating the lymphoma will decrease life expectancy. Based on data from the Surveillance, Epidemiology, and End Results (SEER) database, 23% of patients over the age of 65 receive no treatment at all, and this proportion increased to 33% in patients over the age of 80 (10). Illustrative of this point, at 80 years old, a person in average health has a predicted life expectancy of about 5 years without a lymphoma diagnosis. Consequently, an aggressive NHL is likely the greatest risk to this patient’s life, and treatment should be a consideration in the context of the patient’s goals and wishes. When life expectancy is less than 1 year despite the lymphoma diagnosis, palliative efforts are most appropriate. Historically, performance status and clinical judgment have been used by physicians to attempt to identify those patients who are at greatest risk of toxicity (11,12). However, clinician judgment alone is inadequate in identifying all older patients at risk. Efforts have been made to identify those a greatest risk of toxicitiy (13–15). Comprehensive geriatric assessments (CGAs) are better at delineating those most at risk (16). Utilizing a CGA in DLBCL, Tucci et al. demonstrated that a CGA was better at delineating fit versus unfit in comparison to physician judgment, with about 20% potentially misidentified as fit by physicians (7). In practice, those with the highest risk of toxicity should be considered for dose modifications or reduced-intensity treatments; in this context, geriatric assessments are increasingly being established as important adjuncts to decision making (17).
Comorbidity
In NHL patients over the age of 60 years, 60% to 70% have some comorbidity. The number and severity of comorbidity increases with increasing age in lymphoma patients, and the presence of comorbidities is associated with increased treatment-related mortality, treatment-related toxicity, reduced-intensity treatment, and decreased OS (18–20). Comorbidities specifically assessed by indices of risk, such as the Charlson 221Comorbidity Index, have also been shown to be an independent adverse prognostic factor in patients over age 65 (21,22). Frailty is a distinct physiologic state separate from comorbidity and independently identifies a population at highest risk for toxicity and mortality. Though there is no standardized definition of frailty, it typically is characterized by decreasing physiologic reserves with the inability to adapt to illness or stressors in older patients. It often includes geriatric syndromes, such as falls, incontinence, and delirium, as well as physical symptoms of weakness, slowness, and weight loss. Data from Tucci and the Lymphoma Italian Foundation studies have delineated fit and frail patients, with results indicating the overlap in defining frail and the utility of defining these groups when pursuing curative management (7).
DISEASE-SPECIFIC MANAGEMENT
Diffuse Large B Cell Lymphoma
DLBCL, the most common subtype of NHL, is associated with a median age of 70 years and increasing incidence in older patients; it is fatal without treatment. Front-line anthracycline-based chemoimmunotherapy affords OS rates of 60% to 70% (23,24). Choosing which older patients can tolerate curative therapy requires integration of comorbidity, functional status, and disease characteristics.
TREATMENT
RCHOP chemotherapy is the standard of care. However, this anthracycline-based regimen has been associated with a treatment-related mortality of approximately 6% to 12% and risk of cardiotoxicity in older individuals (23,24). Consequently, the decision to pursue an anthracycline-based regimen must include a thorough evaluation of performance status, comorbidity, and organ function with clinician judgment.
Maneuvers to mitigate toxicity—Prephase
The German Non-Hodgkin Lymphoma Study Group implemented a “pre-phase” treatment of 5 to 7 days of prednisone and a 1-mg dose of vincristine prior to the initiation of chemotherapy, resulting in a 50% reduction in cycle 1 and cycle 2 treatment-related mortality in the RICOVER-60 study (24). Other prospective studies have also suggested a clinical benefit of this strategy (25,26).
Early stage
Early stage I/II disease is highly curable; treatment includes combined modality or chemotherapy alone (27,28). In the pre-rituximab era the SWOG 8736 trial established CHOP × 3 followed by involved field radiation therapy (IFRT) as superior to CHOP × eight cycles in both PFS and OS (29). However, long-term follow-up has shown that this difference ultimately disappears after 8 years (29). Rituximab + CHOP × 3 followed by IFRT in the SWOG 0014 trial in patients with one or more risk factors reported good outcomes, with a PFS of 88% at a median follow-up of 5 years (29). It should be noted that the LYSA group compared RCHOP × four or six cycles with or without radiation using an adapted strategy based on IPI and interim PET, and showed that the combined modality 222was not superior to RCHOP alone (30). The decision to include radiation therapy is made depending on disease location, risk of radiation toxicity, comorbidity, and life expectancy. It should also be considered as a definitive treatment in patients who will not tolerate chemotherapy.
Advanced stage
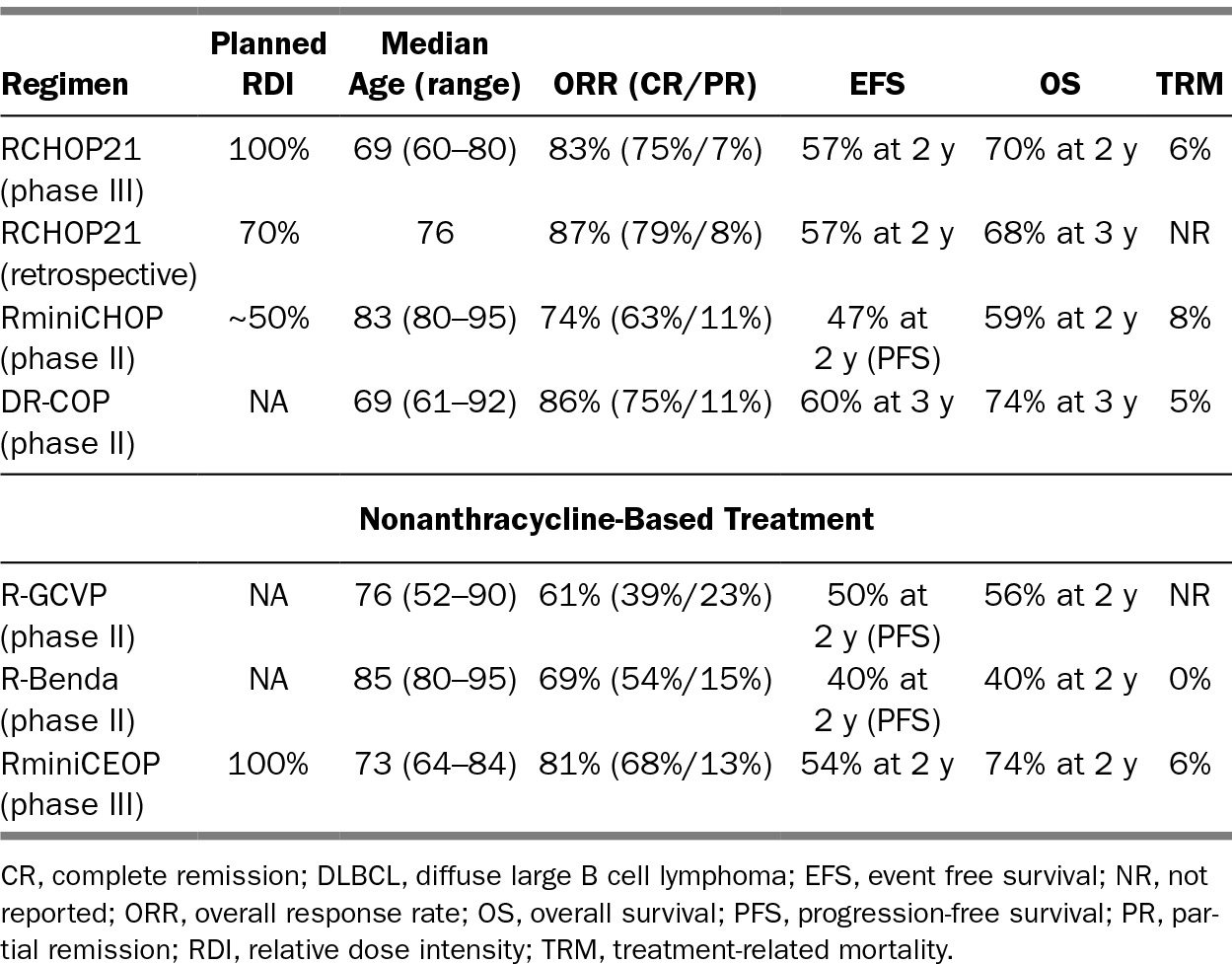
RCHOP every 21 days for six to eight cycles based on the LNH 9805 study is the standard of care established in patients 60 to 80 years old, with significant improvement in 5-year PFS and OS versus CHOP (31). Other trials have examined a dose dense every 14-day regimen or maintenance rituximab strategies and found no improvement in survival, but these trials did demonstrate the importance of rituximab. Higher rituximab density may improve outcomes in elderly males given more rapid clearance (32).
Dose-adjusted (DA) etoposide prednisone oncovin cyclophosphamide doxorubicin (R-EPOCH) is an infusional regimen that may have improved efficacy in certain subsets of DLBCL, including “double hit” (BCL2, MYC by FISH), GCB, and high proliferative rate tumors (MYC expression, high Ki67), and may be less cardiotoxic due to infusional doxorubicin (33,34). However, early reports of the phase III trial comparing DA-R-EPOCH to RCHOP have not confirmed an advantage (35). Nonetheless, this infusional regimen may be attractive in certain older patients given the decreased association with cardiotoxicity.
Reduced intensity/Nonanthracycline
For patients who are poor candidates for full-dose anthracycline-based treatment, there are several reduced therapy options and nonanthracycline-based regimens that retain curative intent. Typically, patients over the age of 80 or those who are frail, or have multiple comorbidities or geriatric syndromes, are not ideal candidates for full-course RCHOP. Trading some efficacy for reduced toxicity is a reasonable strategy in this scenario. A phase II GELA trial evaluated RminiCHOP (relative dose intensity [RDI] of about 50% compared to RCHOP) in patients over the age of 80 and showed a 2-year PFS and OS of 47% and 59%, respectively (5). Infusional doxorubicin and liposomal doxorubicin (Doxil) may be safer options, with less cardiac toxicity, and can be considered in patients where an anthracycline is not an absolute contraindication, but often a nonanthracycline based regimen is preferable (36). Fields et al. demonstrated that a regimen of R-GCVP (rituximab, gemcitabine, cytoxan, vincristine, and prednisone) had a 2-year PFS and OS of 49.8% and 55.8% (6). Other nonanthracycline regimens include R-CEPP, R-CEOP (etoposide replacing doxorubicin), R-CDOP (liposomal doxorubicin), R-bendamustine, and rituximab, cyclophosphamide, mitoxantrone, oncovin, prednisone (R-CNOP) (4,37).
Salvage therapy with high-dose therapy and autologous stem cell rescue (HDT/ASCR) in elderly
In patients who relapse following RCHOP, chemotherapy alone is generally not curative. Second-line chemotherapy followed by HDT/ASCR is the standard of care. For the majority of older patients, HDT/ASCR is not an option due to comorbidity and treatment-related mortality associated with transplant. In the older patient with relapsed disease, the goals become palliative rather than curative. If a patient is transplant ineligible, second-line regimens include clinical trials, R-CVP, rituximab, gemcitabine, oxaliplatin (R-Gem-Ox), rituximab-bendamustine, CEPP, and rituximab with lenalidomide (Figure 23.1) (4–6,38).
223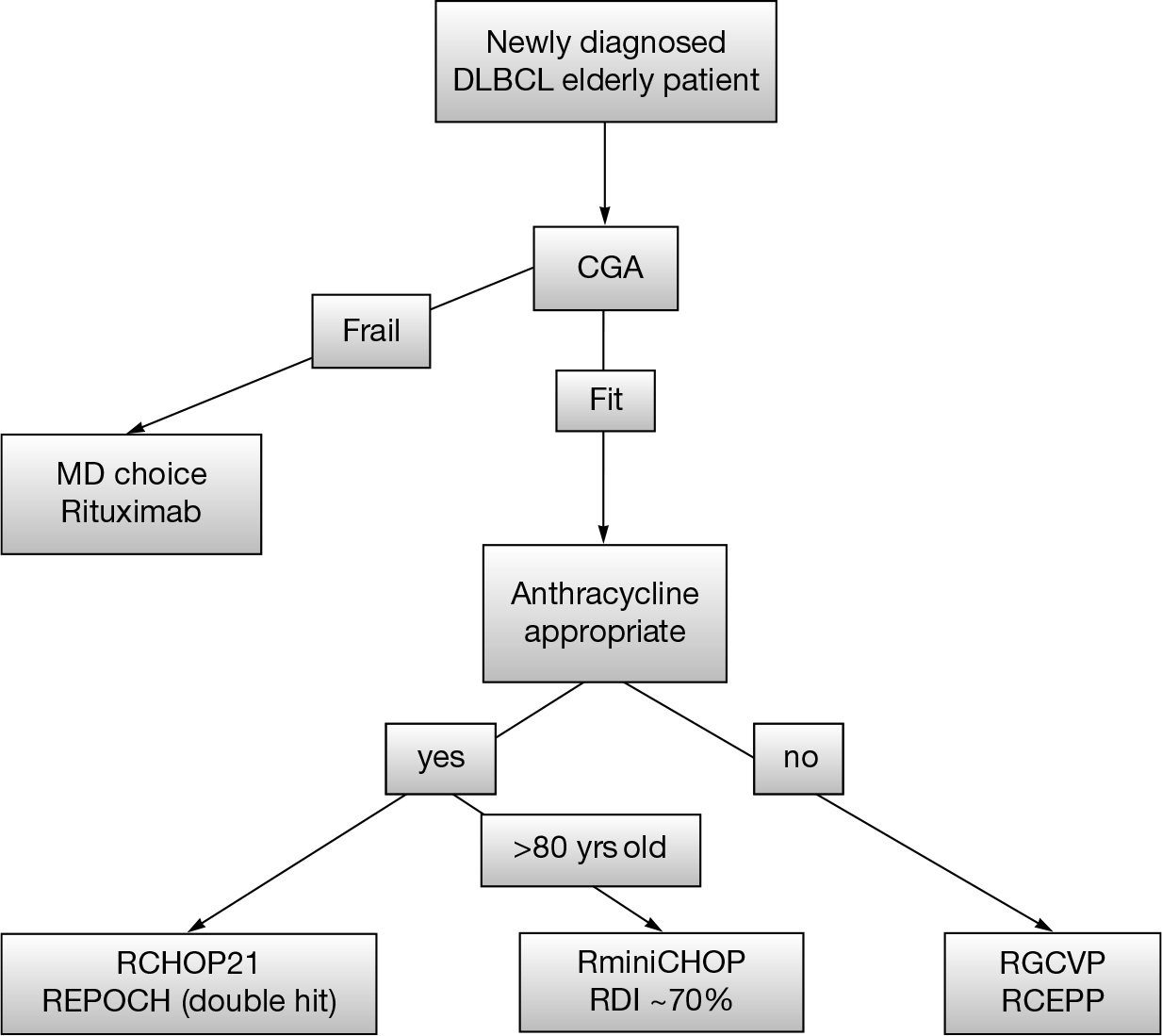
FIGURE 23.1 A suggested clinical algorithm for therapeutic decisions in older DLBCL patients.
DLBCL, diffuse large B cell lymphoma; CGA, comprehensive geriatric assessments; RCEPP, rituximab cyclophosphamide etoposide procarbazine prednisone.
Mantle Cell Lymphoma
MCL is a rare NHL classified as an indolent lymphoma due to its incurability, but with a more aggressive disease behavior and a median survival of 5 to 7 years. The median age of MCL patients is 65, and patients typically present with advanced stage involving lymph nodes, the bone marrow, and gastrointestinal (GI) tract. It has a male predominance of 2:1 and comprises 3% to 10% of NHL in Western countries. A essential feature of MCL is the translocation 11;14, which causes the dysregulation of cell cyclinD1 (rarely D2 or D3). It is characterized by an immunophenotype of CD5(+), CD10(-), CD20(+), CD23(-/+), CD43(+), and Cyclin D1(+) with t(11;14) by FISH. SOX 11 is another marker in MCL that has prognostic 224significance. The presence of a high Ki67%, p53, or MYC expression tends to portend a more aggressive disease course. These are often seen with the blastic MCL subtype, which tends to behave aggressively and yield worse outcomes.
TREATMENT
In select patients, the initial management of older individuals will include consideration for upfront cytarabine-containing induction, followed by HDT/ASCR or R-HyperCVAD (Course A: cyclophosphamide, vincristine, adriamycin, dexamethasone; Course B: methotrexate, cytarabine) given potential PFS improvements over less intensive chemoimmunotherapy. As these approaches are not curative, most older individuals are not recommended this course of action, because of the associated toxicities. Anthracycline-based induction therapy had been one of the standard treatments in MCL (39). RCHOP plus rituximab maintenance is one treatment strategy with duration of responses over 6 years and OS of 87% (40). However, an anthracycline may not be necessary for the treatment of MCL, particularly in older patients who may not tolerate an anthracycline-based regimen. A nonanthracycline-based strategy of rituximab-bendamustine, based on the StiL trial (subgroup) and the BRIGHT trials, is increasingly being used as the initial treatment in older patients. The STiL trial compared RCHOP to bendamustine rituximab (BR) upfront in indolent lymphomas, and MCL demonstrated an improved PFS with BR (69.5 vs. 31.2 months) with less toxicity and better tolerability (40). Similarly, the BRIGHT trial also demonstrated that BR was noninferior to RCHOP/rituximab cyclophosphamide vincristine prednisone (RCVP) with good tolerability (39). With the majority of patients above 60 years in both these trials, BR is a safe and effective treatment with less toxicity and should be considered in older patients, particularly those with significant comorbidity or requiring a nonanthracycline approach. Maintenance rituximab following BR does not appear to improve outcomes.
Biologic-based regimens are also being explored in older patients, such as lenalidomide and rituximab followed by maintenance, and preliminarily are associated with admirable outcomes: ORR 87 and 2-year OS 97% (41). A bortezomib-based regimen—bortezomib with rituximab, cyclophosphamide, doxorubicin, and prednisone (VR-CAP)—had improved PFS (24.7 vs. 14.4 months) versus RCHOP, but no difference in OS (42).
Relapse
There is no standard of care for relapse/refractory MCL. In the recurrent setting, several agents have recently been approved, including ibrutinib (Bruton’s tyrosine kinase inhibitor), lenalidomide (immunomodulatory agent), and bortezomib (proteasome inhibitor). Sequential use of standard chemotherapy is an option as well, depending on the regimen used in the first line. There are also trials that are investigating new combinations of these new agents and chemoimmunotherapy. Other agents with promising activity in MCL include venetoclax, with studies ongoing.
Hodgkin Lymphoma
HL, an entity distinct from NHLs, is the most common lymphoid malignancy in young patients, but possesses a bimodal age distribution with 15% to 35% of patients presenting over the age of 60. Disease biology, comorbidity, functional status, and organ dysfunction can compromise the curability of older HL patients, who often have advanced-stage 225disease, higher risk scores, EBV positivity, and mixed cellularity histology. Based on retrospective data and population databases, older patients with HL (over age 60) have significantly worse outcomes in comparison to younger patients. Although there have been improvements in overall outcomes, this difference continues to persist in prospective studies. For example, in the E2496 study, the cumulative incidence of death was 30% for older patients and 10% for younger at 5 years (43). CALGB 8251 was a prospective trial reporting outcomes with adriamycin bleomycin vinblastine dacarbazine (ABVD): in older patients (≥60 years) 5-year OS was 31%, versus 63% for those aged 40 to 59, and 79% for those less than 40 years old.
TREATMENT
Early stage disease
The standard approach for early stage disease was established by Bonadonna, employing ABVD x4 cycles followed by IFRT (44). Over the years there has been a drive to limit the amount of treatment, to mitigate toxicity while maintaining efficacy. The NCIC HD6 study reported good outcomes with patients treated with ABVD x4 alone if they achieved a CT complete remission (CR) after two cycles of therapy. In early stage favorable disease, the HD10 German Hodgkin study group (GHSG) evaluated ABVD × 2 followed by IFRT at 20 Gy for patients with no risk factors as defined by GHSG; these patients demonstrated OS of 96.6% to 97.5% (45). The advent of PET imaging technology has also helped to limit treatment in early stage patients. The UK RAPID study evaluated ABVD × 3 followed by interim PET; if the PET was negative (Deauville 1-2), then patients were monitored. PET positive patients received an additional cycle of ABVD followed by IFRT (46). Outcomes at 3-year PFS were 93.8% versus 90.7% and OS 97% versus 99.5% for IFRT versus observation respectively, thereby avoiding radiation in 75% of patients (46). In early stage unfavorable disease, the GHSG HD11 trial established ABVD x4 followed by 30 Gy IFRT as an effective treatment option with a 5-year PFS of 87% (47). A full six cycles of ABVD with or without radiation can also be considered, with 5-year PFS of 81% versus 86% with RT, and a 5-year OS of 90% versus 97% with RT (48). Thus, there are several different potential strategies to employ for older patients for early stage HL that take advantage of reduced exposure to chemotherapy and/or radiation, which can be tailored based on the patient. In older, frail patients, radiation alone may be a consideration if there is a concern for significant chemotherapy toxicity, particularly in the context of a latency period of 8 to 12 days for the radiation-related effects.
Advanced stage
Patients with advanced stage HL can be risk-stratified based on the IPS; older patients frequently have higher IPS scores. ABVD is the standard of care for advanced stage HL (44,48,49). Regimens such as Stanford V and bleomycin etoposide adriamycin cyclophosphamide oncovin procarbazine prednisone (escBEACOPP) (HD9 trial) have been compared with ABVD and in older patients are associated with increased toxicity and no improvement in outcomes. Older patients are more vulnerable to bleomycin toxicity, which can at times be fatal. The RATHL trial employed PET imaging after two cycles to risk-adapt therapy in advanced stage HL patients. PET negative patients (Deauville 1-3) were randomized to ABVD versus AVD for 4 additional 226cycles. Outcomes were comparable between ABVD and AVD groups, with PFS 85.4% versus 84.4% and OS 97.1% versus 97.4%, indicating that omitting bleomycin decreased pulmonary toxicity without affecting efficacy in interim negative PET2 patients (50,51). Ongoing efforts are incorporating brentuximab vedotin (BV) into treatment paradigms in lieu of bleomycin.
In patients who cannot receive an anthracycline, other regimens—such as chlorambucil, procarbazine, prednisone, vinblastine (ChLVVP); mustard (nitrogen) oncovin procarbazine prednisone (MOPP) and C-MOPP; vinblastine, cyclophosphamide, procarbazine, etoposide, mitoxantrone, bleomycin, prednisolone (VEPEMB); and PVAG—can be considered (52). Single-agent BV is active in older HL patients, but unlikely to be curative alone (53). In advanced stage disease, the role of radiation is unclear, but in those patients with partial responses or bulk, radiation can be considered and may confer an overall benefit.
Relapse
Salvage chemotherapy followed by HDT/ASCR with or without radiation is the standard of care for patients with relapsed disease. This has resulted in cure in greater than 60% of patients. The applicability of transplant, however, must be assessed in the context of the older patient. More recently, BV has shown single-agent activity in relapsed disease posttransplant with ORR of 75% and a CR of 34% and is approved for failure after HDT/ASCT or failure of at least two prior chemotherapy regimens in patients who are not transplant candidates. BV should be considered in older patients with relapsed HL, particularly if they are not candidates for transplant, but with close attention to peripheral neuropathy.
Immunotherapy with PD1 inhibitors (nivolumab, pembrolizumab) has been an exciting area of research with high response rates. In phase I trials with nivolumab in relapse/refractory HL, overall response rates were 87% and CR of 17% (54). Similarly, relapsed/refractory patients treated with pembrolizumab (KEYNOTE-013) showed ORR 66% with 21% CR (55). Nivolumab is now FDA approved for HL. Ongoing trials are investigating the role of antibody drug conjugate (ADC) and immunotherapy in the relapsed and upfront setting and in combination.
Indolent Lymphomas
Indolent lymphomas include follicular Grade 1, 2, 3a and MZL (splenic, nodal, and extranodal), lymphoplasmacytic lymphoma, and WM. Because of their indolent clinical behavior, patients often do not require treatment at diagnosis. There are several options for treatment of indolent lymphomas, and there are specific National Comprehensive Cancer Nerwork/Groupe d’Etude des Lymphomes Folliculaires (NCCN/GELF) criteria that help decide when treatment is indicated. These include cytopenias, organ dysfunction, B symptoms, bulky disease (single mass > 7 cm or 3 or more masses > 3 cm), splenomegaly, and rate of disease progression. If a patient is asymptomatic and does not have the aforementioned criteria, then watchful waiting is appropriate. Importantly, in patients over the age of 70 diagnosed with an indolent lymphoma who undergo watchful waiting, 40% never require treatment.
227If treatment is required, there are many options, which include single-agent rituximab, combination chemoimmunotherapy (BR, RCVP, RCHOP), radioimmunotherapy, and newer agents (idelesalib, lenalidomide). Given the many therapeutic options and long disease course, treatment decisions can more easily be matched to patient tolerability. The aforementioned StiL and BRIGHT trials have indicated that in indolent lymphomas, BR is noninferior, or even superior, to RCHOP with less toxicity (39,40). Patients with progressive disease within 24 months of chemoimmunotherapy may have a more aggressive disease course (56).
TAKE HOME POINTS
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree