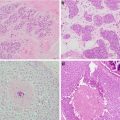
Fig. 2.1
Mammogram showing microcalcifications under nipple in a 45-year-old patient with ductal carcinoma in situ
In some centers, even patients with locally advanced breast cancer are considered eligible for nipple sparing. In a recent study of 139 patients with stage 2B or stage 3 breast cancer treated with NSM, only 5% of patients developed an isolated local recurrence at a mean follow-up of 41 months and no recurrence involved the retained nipple areola complex [23]. The authors concluded that with appropriate multimodality therapy, nipple sparing does not increase risk of local recurrence, even in patients with locally advanced disease .
Patients who have had prior radiation or who need post-mastectomy radiation are also candidates for NSM. Although prior radiation and post-mastectomy radiation increase risk for complications with any reconstruction, most patients who undergo NSM with radiation do well [24]. Tang and colleagues at our institution compared 816 NSM with no radiation to 69 NSM in previously irradiated breasts [24]. Prior radiation increased the rate of skin necrosis from 4.5 to 11.6% and increased risk of total nipple necrosis from 0.9 to 4.3% [24]. Among 97 NSM that received post-mastectomy radiation, 10.3% had skin necrosis and 4.1% had total nipple necrosis. Rates of implant loss were 2.2% without radiation, 2.9% with prior radiation, and 8.2% with post-mastectomy radiation [24]. Risk factors for complications with radiation included smoking, age >55, breast volume >800 cm3, and periareolar incision placement [24]. It was concluded that prior radiation or the need for post-mastectomy radiation are not absolute contraindications to NSM and that complications could be minimized with appropriate patient selection [24].
Cosmetic factors are also considered when assessing eligibility for NSM. Nipple sparing is contraindicated in patients for whom the retained nipple would be in an unacceptable position on the reconstructed breast. Marked breast ptosis or very large breast size may result in poor nipple position if the nipple is preserved. Salgarello and colleagues advise against NSM in patients with bra size larger than a D cup and for D cup breasts with grade 3 ptosis, that is, ptosis with the nipple well below the inframammary fold [25]. In addition, an excised breast weight of more than 750 g and a sternal notch-to-nipple distance of greater than 26 cm have been found to increase skin flap complications in patients undergoing skin-sparing mastectomies, so caution with use of NSM is also advised in these patients [26].
Nipple Mastectomy for Risk Reduction
NSM for risk reduction is endorsed by the most recent NCCN guidelines [27] (Fig. 2.2). Risk reduction surgery should be considered in women with a known BRCA 1 or BRCA 2 mutation, or another gene mutation with a significantly increased risk of breast cancer , or a compelling family history of breast cancer [27]. In a retrospective analysis of 639 women treated with bilateral prophylactic subcutaneous mastectomy from 1960 to 1993 for a family history of breast cancer, Hartmann and colleagues noted a 90% reduction in breast cancer at 14-year median follow up [4]. Twenty-six of these patients were later found to have BRCA 1 or BRCA2 mutations; 23 had been treated with subcutaneous mastectomy and three with simple mastectomy [28]. No cancers had developed at 13.4-year median follow-up among these BRCA mutation carriers. Using published data for the likelihood of breast cancer in BRCA1 and BRCA2 mutation carriers, they would have expected six to nine breast cancers to develop during the follow-up period with no intervention. Their results suggest that the risk of breast cancer is reduced by 89.5–100.0% in BRCA1 and BRCA2 mutation carriers after prophylactic subcutaneous mastectomy [28]. With current NSM techniques that excise all subareolar tissue, even greater risk reduction may be possible.
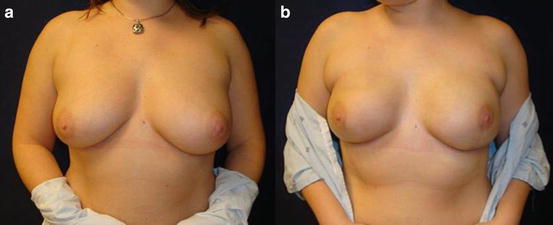

Fig. 2.2
Prophylactic NSM with single-stage direct to implant in BRCA mutation carrier with inferolateral incision, preop (a) and 1 month postop (b)
Yao et al. reported on NSM in 397 breasts of 201 BRCA1 and BRCA2 carriers, 125 had a BRCA1 mutation, and 76 had a BRCA2 mutation; 150 (74.6%) patients underwent NSM solely for risk reduction and 51 (25.4%) underwent NSM for unilateral cancer and contralateral risk reduction [29]. Incidental cancers were found in four (2.7%) of the 150 risk reduction patients and two (3.9%) of the 51 cancer patients. The nipple areola complex was involved with cancer in three (5.8%) of the cancer patients. No prophylactic mastectomy had a positive nipple margin. With a mean follow-up of 32.6 months, no patient developed a recurrence at the NAC, although four patients (three cancer patients and one prophylactic) experienced cancers elsewhere – two locally and two in the axilla [29]. When Peled and colleagues reviewed outcomes of 26 BRCA mutation carriers who underwent NSM for risk reduction and 27 BRCA mutation carriers who underwent NSM for unilateral breast cancer and contralateral risk reduction, they found no evidence of new or recurrent cancers, respectively, at 51-month mean follow-up [30]. In a recent multi-institutional study of risk-reducing NSM in 348 patients with BRCA mutations, Jakub et al. reported no evidence of cancer at 56-month mean follow-up [31]. Although follow-up in NSM series is limited, to date NSM appears to be a safe approach for risk reduction and for cancer treatment in high-risk patients .
Oncologic Outcome of Therapeutic Nipple-Sparing Mastectomy
Despite increasing use of NSM in the last 5–10 years, concerns remain about oncologic safety given the lack of long-term follow-up . Sites potentially at risk for new or recurrent breast cancer after NSM include the retained nipple areola complex and skin flaps, particularly at the periphery of the breast where visualization may be difficult with incisions used for NSM. Review of 297 NSM for cancer at Massachusetts General Hospital found a 3.0% risk of locoregional recurrence at 42-month median follow-up, with no recurrence involving the retained nipple areola complex [32]. These results are consistent with oncologic outcomes in other NSM series reported over the last 5 years, summarized in Table 2.1. Locoregional recurrence rates after NSM range from 0 to 4.6% at 10–60 months of follow-up [14, 15, 21, 22, 30, 33–36].
Table 2.1
Oncologic outcomes of nipple-sparing mastectomies for cancer
Author | Institution | Year | # Breasts | Follow-up (months) | Locoregional recurrence (%) | NAC recurrence |
|---|---|---|---|---|---|---|
Orzalesi | Italian Nat’l Database | 2016 | 755 | 36 | 2.9 | 5 |
Krajewski | Mayo Clinic | 2015 | 226 | 24 | 1.7 | 0 |
Coopey | Mass General | 2013 | 156 | 22 | 2.6 | 0 |
Lohsiriwat | European Inst. of Oncology | 2012 | 861 | 50 | 4.2 | 7 |
Peled | UCSF | 2012 | 412 | 28 | 2 | 0 |
Boneti | University AR | 2011 | 152 | 25 | 4.6 | 0 |
Filho | MSKCC | 2011 | 157 | 10 | 0 | 0 |
Jenson | John Wayne | 2011 | 127 | 60 | 0 | 0 |
Only two modern studies report any tumor recurrences in the retained nipple after NSM. Lohsiriwat and colleagues from Italy performed 861 NSM for cancer and seven patients (0.8%) developed recurrences in the nipple areola complex with 50 months median follow-up [35]. The mean time to nipple recurrence was 32 months, and in all cases the nipple areola complex was removed. At a mean of 47 months after nipple removal, no patient developed any further local or distant recurrence. Lohsiriwat et al. describe leaving at least 5 mm of glandular tissue beneath the nipple areola complex to prevent necrosis and giving a single dose of radiation to the nipple areola complex and 1 cm beyond, a technique which is different than those used in North American studies [35]. Recently, Orzalesi et al. reviewed a national multi-institutional registry of NSM performed in Italy which included 1,006 patients [36]. Of 755 cases included in the locoregional recurrence analysis, 5 (0.7%) developed a recurrence of the nipple areola complex at a mean 36-month follow-up. In this series, no particular technique was described for dissection under the nipple areola complex [36].
In a review of 20 NSM series, De La Cruz and colleagues found disease-free survival in series with <3-year follow-up, 3–5-year follow-up, and >5-year follow-up was 93.1%, 92.3%, and 76.1%, respectively [37]. Many of the studies with >5 years of follow-up included patients treated in the mid-1980s and 1990s [38, 39]. More recent retrospective cohort studies comparing NSM plus immediate reconstruction to mastectomy without reconstruction have shown no significant difference in local or distant recurrence rates with NSM [40, 41]. When Adam et al. matched 67 NSM plus reconstruction patients to 203 mastectomy without reconstruction patients, they found no significant difference in estimated 5-year disease-free survival (94.1 and 82.5%, p = 0.068) or in overall survival (OS) (96.2 and 91.3%, p = 0.166) between them [40]. In fact, they noted a nonsignificant trend toward worse outcomes in the mastectomy without reconstruction group and concluded that NSM with reconstruction was a safe alternative. Similarly, when Park and colleagues compared 114 patients who underwent skin-sparing or nipple-sparing mastectomy plus immediate reconstruction to a matched control group of patients who underwent mastectomy with no reconstruction, they found no significant difference in 5-year locoregional recurrence-free survival between the two groups , 96.4% and 96.1%, respectively (p = 0.552) [41].
Surgical Technique
Incision Placement
The ideal incision for NSM would allow thorough resection of all of the breast tissue by the breast surgeon and ease of reconstruction for the plastic surgeon, through an aesthetically favorable scar [42]. In addition, preservation of nipple and areola blood supply is critical to successful nipple sparing. Following mastectomy, blood is supplied to the NAC from the periphery of the breast through the subdermal plexus in the skin flaps. Incisions used for NSM must preserve inflow to the nipple.
There are six basic types of incisions for NSM: inferolateral, inframammary, lateral radial, inferior radial, periareolar, and through extension of a prior scar. With the inferolateral incision, the incision begins on the lateral border of the breast at the same horizontal level as the nipple (3 o’clock position on the left breast and 9 o’clock position on the right breast). The incision is curved inferomedially along the outer border of the breast. The incision continues medially until it intersects with an imaginary vertical line through the nipple at the 6 o’clock position [42] (Fig. 2.3). The incision length is typically 10–12 cm and must be long enough to accommodate the surgeon’s hand. This incision preserves the internal mammary perforators that supply the medial skin flaps and provides easy access to the axilla. The inferolateral incision provides a favorable cosmetic result, but its inferior location can make dissection of the superomedial breast more challenging [43]. Access to the superior and medial aspects of the breast is facilitated by separating the breast from the pectoralis muscle.
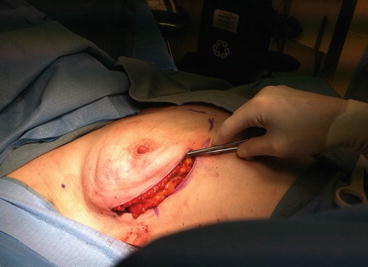

Fig. 2.3
Inferolateral incision placement
The inframammary fold incision remains in the inferior portion of the breast along the inframammary fold, centered beneath the nipple, and spans 12–14 cm [44]. It is useful for DIEP flap reconstructions as it provides good medial exposure for the anastomosis of the deep inferior epigastric vessels to the internal mammary vessels. This incision also has the cosmetic advantage of being completely hidden by the breast in the upright position. It has the disadvantage of giving a more challenging exposure and usually requires a longer scar, which could interfere with blood supply to the nipple or inferior skin flap [42]. Another disadvantage of this incision is that a separate axillary incision is usually required for axillary staging [44].
The lateral radial and inferior radial incisions are more similar to incisions used for standard skin-sparing mastectomies and may be technically easier than the inferolateral and inframammary incisions. However, both leave visible scars prominent on the breast, and the lateral radial incision has been known to cause deviation of the nipple toward the scar [45]. On the other hand, an inferior radial incision is sometimes favored if a patient has larger breasts and would benefit from skin excision to raise the nipple to a more superior position on the reconstructed breast. A periareolar incision placed either along the superior or inferior half of the areola and usually with a lateral radial extension can also be utilized [43]. This approach may be technically easier for the surgeon as it is similar to the traditional skin-sparing mastectomy approach. Not surprisingly, however, placing an incision along the edge of the areola has been associated with a higher risk of nipple necrosis and nipple loss. Some authors believe that incisions in this location should be avoided, although others have found it an acceptable approach [33, 46].
Overview of Technique
Decisions about incision placement should be made in collaboration with the plastic surgeon and, to the extent possible, take patient preference into account. At our institution the majority of NSM are performed via an inferolateral approach ; this allows the mastectomy and sentinel lymph node biopsy or axillary dissection to be performed through a single incision. Inferolateral and inferior incisions require modifications in technique compared to standard skin-sparing mastectomy incisions which are more centrally located on the breast. In our inferolateral approach, after the incision is made, a short flap is raised to the chest wall inferiorly and laterally, preserving the inframammary fold. If a sentinel lymph node biopsy with frozen section is planned, dissection can then be continued along the lateral border of the breast and serratus anterior muscle until the axillary fat pad is encountered. The sentinel lymph node can then be obtained, and frozen section performed while the mastectomy is completed.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree