Fig. 8.1
(a) Flat epithelial atypia (FEA ) (b) Atypical ductal hyperplasia (ADH )
The immediate question a clinician faces when a patient is diagnosed with pure FEA on core biopsy is whether surgical excision is necessary. In the literature, reported upgrade rates vary widely, ranging from 0 to 21% [6, 9–16] (Table 8.1). All the studies are limited by a retrospective design, and many also have a small sample size. The published recommendations for treatment are likewise variable. Some recommend routine excision for all FEA patients similar to when ADH is diagnosed on core biopsy . Alternatively, some argue for case-by-case decision-making that would factor in imaging findings (such as the presence of residual calcifications in the post-biopsy mammogram) as well as factors such as a personal history of breast cancer. This case-by-case process would allow for some patients to undergo imaging surveillance rather than immediate excision. Although no prospective, randomized data is available to definitively answer this question of excision vs. surveillance, there is some published data on patients who have not undergone immediate excision. These patients have not developed a subsequent breast cancer at the site of their FEA biopsy within the follow-up periods [6, 10, 13, 16].
Table 8.1
Reported upgrade rates for FEA
Article | Number of pure FEA cases/number excised | Upgrades to DCIS or IC | Indication for biopsy | Residual lesion post-biopsy | Patients without excisions |
|---|---|---|---|---|---|
Kunju and Kleer (2007) | 14/14 | 3 (21%) | Calcifications | Unknown | N/A |
Noel et al. (2009) | 62/20 | 0 | Calcifications | Present in the 20 excised cases | No changes in mammograms at 6–12 months post biopsy |
Chivukula et al. (2009) | 39/35 | 5 (14%) | Calcifications | Unknown | No follow-up provided |
Lavoue et al. (2011) | 60/60 | 8 (13%) | Calcifications, mass | Present in at least 42a | N/A |
Uzoaru et al. (2012) | 145/95 | 3 (3%) | Calcifications, mass | Unknown | No changes in mammograms with mean follow-up of 5 years |
Peres et al. (2012) | 128/95 | 9 (9%)c | Calcifications, mass | Unknown | No changes in mammograms with median follow-up of 13 months |
Khoumanis et al. (2013) | 104/94 | 10 (10%) | Calcifications, mass | Unknown | No changes in mammograms with mean follow-up of 36 months |
Prowler et al. (2014) | 24/24 | 0 | Calcifications, mass, MRI enhancement | Unknown | N/A |
Calhoun et al. (2015) | 73/73 | 5 (7%) | Calcifications, mass, MRI enhancement | 14 completely removed at biopsyb | N/A |
Atypical Ductal Hyperplasia
Atypical ductal hyperplasia (ADH ) morphologically resembles low-grade ductal carcinoma in situ (DCIS ). Both are composed of a proliferation of monomorphic epithelial cells that are evenly spaced with distinct cell borders. Unlike FEA , the proliferating cells can be solid or have architecture and form bridges or micropapillae. Given the morphologic overlap, quantitative criteria are often used to distinguish ADH from low-grade DCIS . If the proliferation completely involves of at least two membrane-bound spaces or has a size greater than 2 mm, these lesions would be diagnosed as DCIS rather than ADH [17]. Some authors have suggested a conservative approach to low-grade ductal proliferations on core biopsy and will classify those measuring up to 3 mm as ADH [18]. This avoids overdiagnosing small lesions as low-grade DCIS by leaving the final determination of extent and therefore classification to the excision specimen rather than deciding based on the core biopsy findings alone.
Given the difficultly in accurately distinguishing ADH from low-grade DCIS on core biopsy alone, surgical excision is routinely recommended for ADH . The upgrade rates for ADH at excision are in the range of 10–20%.
Atypical Lobular Hyperplasia and Classic Lobular Carcinoma In Situ
Classic lobular neoplasia encompasses both atypical lobular hyperplasia (ALH ) and lobular carcinoma in situ (LCIS ). The cells in ALH and classic LCIS have identical morphology. Both lesions are composed of dyscohesive small, round cells with uniform nuclei that are often eccentrically placed. Cytoplasmic vacuoles are common. The difference between the two diagnoses comes with the degree of lobular involvement and/or distension by these cells. In comparison to ALH (Fig. 8.2a), classic LCIS shows greater distention of the acini within the TDLU as well as more complete involvement of the TDLU itself (Fig. 8.2b).
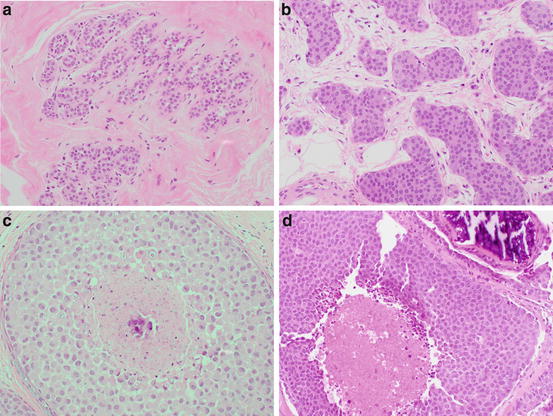

Fig. 8.2
(a) ALH (b) classic LCIS (c) pleomorphic LCIS (d) LCIS with necrosis
Lobular neoplasia is considered both a risk factor and a non-obligate precursor for developing invasive carcinoma in either breast. Cohort studies have shown that the relative risk of developing breast cancer for women with ALH or classic LCIS is estimated at 4 and 10×, respectively [19]. A minority of women with classic lobular neoplasia subsequently develops invasive breast cancer; no clinical or pathological features can accurately predict which women are at risk for progression.
ALH and classic LCIS are most often incidental findings in core biopsies, and the question of whether surgical excision of the biopsy site is necessary is still controversial. The reported upgrade rates in the literature are highly variable, and many studies have significant limitations including the selection bias inherent in their retrospective design. Many also fail to include crucial information about pathologic-radiologic concordance. Discordant cases such as classic LN associated with a mass on imaging should undergo excision to exclude an invasive component that was not sampled in the core biopsy . Unfortunately, discordant cases are sometimes reported as true “upgrades” in the literature, creating a falsely elevated upgrade rate. There is also the question of whether morphologic variants such as pleomorphic LCIS and necrotic LCIS were included for analysis in some studies. Retrospective studies that included pathologic review and imaging concordance had reported upgrade rates of 1.3–4% [20–22]. Prospective studies that included only women with classic lobular neoplasia (ALH and/or LCIS , no LCIS variants) diagnosed on core biopsy with concordant imaging findings also show an upgrade rate of 3–4% [23–25] (Table 8.2). Therefore, when ALH or classic LCIS is an isolated incidental finding on core biopsy , radiologic-pathologic correlation is recommended to determine management. In contrast, patients with an associated mass, ductal atypia (such as ADH ) with the same core biopsy , or a variant of LCIS should undergo excision.
Table 8.2
Upgrade rates for prospectively excised classic lobular neoplasia in the recent literature
Study | Number of upgrades | Upgrade rate (%) |
|---|---|---|
Rendi (2012) | 3/68 | 4.4 |
Murray (2013) | 2/72 | 3.0 |
Nakhlis (2016) | 2/77 | 3.0 |
Susnik (2016) | 7/180 | 3.9 |
Total | 14/397 | 3.5 |
Variants of Lobular Carcinoma In Situ
It is important to be aware of the morphologic variants of LCIS : pleomorphic and necrotic types. These are uncommon entities and in the past have likely been diagnosed and treated as DCIS [26]. Pleomorphic LCIS (P-LCIS ) is composed of variably dyscohesive cells with nuclei 3–4× the size of a lymphocyte that are often eccentrically placed with prominent nucleoli [27] (Fig. 8.2c). Necrosis is sometimes present in P-LCIS but not required for the diagnosis. In contrast, identifying necrosis is necessary for the diagnosis of necrotic LCIS (N-LCIS ) which is otherwise composed of cells with similar cytologic features to classic LCIS with more marked distension of the involved acini [28]. This necrosis can be puntate or comedo type. The presence of associated necrosis that undergoes calcification with either P-LCIS or N-LCIS leads to mammographic similarities with DCIS [29]. Although the aggressiveness of these variants is unknown, some studies have shown a higher association with invasive carcinoma. Therefore, in contrast to classic LCIS , the current recommendation for LCIS variants is surgical excision.
The Importance of Pathologic-Radiologic Concordance
Pathologic-radiologic concordance has been emphasized in the discussion of each category of atypia above, but how does one decide when it has been achieved? Multiple parameters come into making this assessment, and they vary from patient to patient. The most obvious question that needs to be answered after every core biopsy is: do the pathologic findings correlate with the imaging impression? Although the question itself is obvious, getting to the answer involves assessing multiple parameters. All the atypias discussed in this chapter are not by themselves mass-forming lesions, but they may secondarily involve a mass (such as when ALH is identified within a fibroadenoma). FEA and ADH are often associated with the calcifications that were the target of the core biopsy . ALH and classic LCIS are most often a completely incidental finding, although they too can be associated with calcifications. When a core biopsy is performed targeting a mass seen on mammography and/or ultrasound and the resultant cores show only FEA , ALH /LCIS , or ADH , the pathologic-radiologic findings may be discordant. Not only the type of lesion identified on breast imaging but the level of suspicion assigned to that lesion by the radiologist should be taken into account. The imaging workup that precedes obtaining a core biopsy provides an overall assessment of the lesion using the Breast Imaging Reporting and Data System (BI-RADS). BI-RADS 4 lesions are considered suggestive of malignancy, and BI-RADS 5 lesions are highly suggestive of malignancy; biopsy is recommended for both categories (https://www.acr.org/). When a core biopsy is performed, targeting a finding categorized as BI-RADS 5 and the resultant cores show only atypia (ductal or lobular); the pathologic-radiologic findings may be discordant. The adequacy of the sample should also be considered, and can be evaluated by variables such as the number of cores obtained and the extent of the lesion removed by the core biopsy . Clinical variables such as age, breast cancer risk, and findings on physical examination may also factor into deciding whether or not surgical excision should be recommended.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree