Hormone receptor-positive/HER2-negative
HER2-positive
Triple-negative
CDK inhibitors
Palbociclib with bazedoxifene in HR-positive breast cancer
NCT02448771
CDK4/CDK6 inhibitor, ribociclib (LEE011), in combination with trastuzumab or T-DM1 for HER2-positive advanced/MBC
NCT02657343
Palbociclib with bicalutamide for the treatment of androgen receptor-positive MBC
NCT02605486
Palbociclib with bicalutamide for the treatment of androgen receptor-positive MBC
NCT02605486
PD-0332991 in combination with T-DM1 in the treatment of patients with advanced HER2-positive BC
NCT01976169
Sapacitabine and seliciclib in patients with advanced solid tumors
NCT00999401
Sapacitabine and seliciclib in patients with advanced solid tumors
NCT00999401
Dinaciclib with pembrolizumab in patients with advanced BC and assessment of MYC oncogene overexpression
NCT01676753
PI3K/mTOR inhibitors
MLN0128 with exemestane or fulvestrant in postmenopausal women with ER/PR+ MBC
NCT02049957
Everolimus, letrozole, and trastuzumab in HR- and HER2-positive patients
NCT02152943
Eribulin and everolimus in patients with triple-negative MBC
NCT02120469
Tamoxifen plus goserelin with alpelisib (BYL719) or buparlisib (BKM120) in premenopausal patients with HR-positive/HER2-negative, locally advanced, or MBC
NCT02058381
BYL719 and T-DM1 in HER2-positive MBC with progression on prior trastuzumab- and taxane-based therapy
NCT02038010
Dose escalation of MK-2206 with weekly paclitaxel in patients with locally advanced or metastatic solid tumors with an expansion in advanced BC
NCT01263145
BYL719 and nab-paclitaxel in locally recurrent or metastatic HER2-negative breast cancer
NCT02379247
Copanlisib in combination with trastuzumab in HER2-positive breast cancer
NCT02705859
BYL719 and nab-paclitaxel in locally recurrent or metastatic HER2-negative breast cancer
NCT02379247
AZD2014 plus fulvestrant in patients with estrogen receptor-positive MBC
NCT01597388
LJM716, BYL719, and trastuzumab in patients with HER2+ MBC
NCT02167854
AZD2014 with selumetinib in patients with advanced cancers
NCT02583542
GDC-0032 with either docetaxel or paclitaxel in patients with HER2-negative, locally advanced, or metastatic breast cancer or NSCLC
NCT01862081
Taselisib (GDC-0032) in combination with anti-HER2 therapies in participants with advanced HER2+ breast cancer
NCT02390427
GDC-0032 with either docetaxel or paclitaxel in patients with HER2-negative, locally advanced, or MBC or NSCLC
NCT01862081
Ascending doses of AZD5363 under adaptable dosing schedules in patients with advanced solid malignancies
NCT01226316
Ascending doses of AZD5363 under adaptable dosing schedules in patients with advanced solid malignancies
NCT01226316
PQR309 and eribulin in metastatic HER2-negative and triple-negative breast cancer
NCT02723877
AZD5363 combined with paclitaxel in breast cancer patients
NCT01625286
Lapatinib, everolimus, and capecitabine for HER2-positive MBC with CNS progression after trastuzumab
NCT01783756
AZD5363 combined with paclitaxel in breast cancer patients
NCT01625286
MK2206 with anastrozole, fulvestrant, or anastrozole and fulvestrant in postmenopausal women with MBC
NCT01344031
LY2780301 in combination with weekly paclitaxel in HER2-negative MBC
NCT01980277
Dose escalation of MK-2206 with weekly paclitaxel in patients with locally advanced or metastatic solid tumors with an expansion in advanced breast cancer
NCT01263145
PQR309 and eribulin in metastatic HER2-negative and triple-negative breast cancer
NCT02723877
LY2780301 in combination with weekly paclitaxel in HER2-negative MBC
NCT01980277
Combined CDK and PI3K/mTOR inhibition
LEE011 with everolimus and exemestane in HR-positive HER2-negative advanced breast cancer
NCT01857193
Ribociclib with everolimus + exemestane in HR+ HER2− locally advanced/MBC post progression on CDK4/CDK6 inhibitor
NCT02732119
LEE011 with fulvestrant and BYL719 or BKM120 in advanced breast cancer
NCT02088684
LEE011 and BYL719 with letrozole in adult patients with advanced ER+ breast cancer
NCT01872260
LEE011 with buparlisib and letrozole for HR+, HER2-negative postmenopausal women with locally advanced or MBC
NCT02154776
Palbociclib with everolimus and exemestane in ER-positive HER2-negative MBC
NCT02871791
AZD2014 and palbociclib on a background of hormonal therapy in patients with locally advanced/metastatic ER-positive breast cancer
NCT02599714
GDC-0077 as a single agent and in combination with endocrine and targeted therapies in locally advanced or metastatic PIK3CA-mutant HR-positive breast cancer
NCT03006172
Gedatolisib with palbociclib and either letrozole or fulvestrant in metastatic or locally advanced/recurrent breast cancer
NCT02684032
Combined IGFR and mTOR inhibition
BI 836845 and everolimus in combination with exemestane in women with HR+/HER2− advanced breast cancer
NCT02123823
FGFR inhibitors
AZD4547 with either anastrozole or letrozole in ER+ patients who have progressed on treatment with anastrozole or letrozole
NCT01791985
Dose-escalation study of INCB054828 in subjects with advanced malignancies
NCT02393248
Dose-escalation study of INCB054828 in subjects with advanced malignancies
NCT02393248
JAK inhibitors
Ruxolitinib in combination with weekly paclitaxel in HER2-negative MBC
NCT02041429
Ruxolitinib in combination with trastuzumab in HER2-positive MBC
NCT02066532
Ruxolitinib in combination with weekly paclitaxel in HER2-negative MBC
NCT02041429
Kinase receptor inhibitors
Trastuzumab emtansine (T-DM1) with neratinib in HER2-positive MBC
NCT02236000
Galunisertib, LY2157299 (TGFβR1 inhibitor), with paclitaxel in patients with androgen receptor-negative, triple-negative MBC
NCT02672475
KD019 (tesevatinib) and trastuzumab in subjects with HER2-positive MBC
NCT02154529
Immune therapy
Anti-PD-L1, MEDI4736, with tremelimumab in subjects with advanced solid tumors
NCT01975831
Durvalumab in patients with HER2-positive MBC receiving trastuzumab
NCT02649686
Pembrolizumab plus chemotherapy in triple-negative MBC
NCT02734290
Mesothelin-specific chimeric antigen receptor-positive T cells in patients with mesothelin-expressing MBC
NCT02792114
Anti-PD-1 monoclonal antibody (MK-3475) in advanced, trastuzumab-resistant, HER2-positive breast cancer
NCT02129556
Eribulin with pembrolizumab in subjects with triple-negative MBC
NCT02513472
Nivolumab with nab-paclitaxel plus or minus gemcitabine in pancreatic cancer, nab-paclitaxel/carboplatin in stage IIIB/IV NSCLC or nab-paclitaxel in recurrent MBC
NCT02309177
Pembrolizumab and monoclonal antibody therapy (T-DM1 or trastuzumab) in patients with advanced cancer
NCT02318901
Pembrolizumab with INCB039110 (JAK inhibitor) and/or pembrolizumab with INCB050465 (PI3K-delta inhibitor) in advanced solid tumors
NCT02646748
Entinostat, nivolumab, and ipilimumab in patients with solid tumors that are metastatic or cannot be removed by surgery or HER2-negative, locally advanced, or MBC
NCT02453620
Atezolizumab with trastuzumab emtansine or with trastuzumab and pertuzumab in HER2-positive breast cancer
NCT02605915
Entinostat, nivolumab, and ipilimumab in patients with solid tumors that are metastatic or cannot be removed by surgery or HER2-negative, locally advanced, or MBC
NCT02453620
Adenoviral transduced autologous dendritic cell vaccine expressing HER2/Neu ECTM in adults with tumors with 1–3+ HER2/Neu expression
NCT01730118
Varlilumab (CDX-1127, anti-CD27 agonist) with atezolizumab (MPDL3280A, anti-PD-L1) in patients with advanced cancer
NCT02543645
Chimeric antigen receptor-modified T cells for HER2-positive recurrent and MBC
NCT02547961
Avelumab with other immunotherapies in advanced malignancies (avelumab plus PF-05082566, anti-4-1BB antibody, in triple-negative cohort)
NCT02554812
Nivolumab with nab-paclitaxel plus or minus gemcitabine in pancreatic cancer, nab-paclitaxel/carboplatin in stage IIIB/IV NSCLC, or nab-paclitaxel in recurrent MBC
NCT02309177
Durvalumab with paclitaxel in patients with triple-negative PD-L1 positive MBC
NCT02628132
PARP inhibitors
Veliparib and carboplatin in patients with HER2-negative MBC
NCT01251874
Veliparib and carboplatin in patients with HER2-negative MBC
NCT01251874
Combined immune therapy and PARP inhibition
Durvalumab with olaparib in advanced solid tumors, including TNBC
NCT02484404
Niraparib with pembrolizumab in patients with triple-negative breast cancer or ovarian cancer
NCT02657889
HDAC inhibitors
ACY-1215 (ricolinostat) with nab-paclitaxel in unresectable or MBC
NCT02632071
ACY-1215 (ricolinostat) with nab-paclitaxel in unresectable or MBC
NCT02632071
ACY-1215 (ricolinostat) with nab-paclitaxel in unresectable or MBC
NCT02632071
Panobinostat (LBH589) and letrozole in patients with MBC
NCT01105312
Entinostat, lapatinib, and trastuzumab in patients with locally recurrent or distant relapsed MBC previously treated with trastuzumab only
NCT01434303
Panobinostat (LBH589) and letrozole in patients with MBC
NCT01105312
Ixabepilone and vorinostat in MBC
NCT01084057
Ixabepilone and vorinostat in MBC
NCT01084057
Ixabepilone and vorinostat in MBC
NCT01084057
Romidepsin plus cisplatin in locally recurrent or metastatic triple-negative breast cancer
NCT02393794
Antibody-drug conjugates (ADC)
SGN-LIV1A in breast cancer patients
NCT01969643
SGN-LIV1A in breast cancer patients
NCT01969643
SGN-LIV1A in breast cancer patients
NCT01969643
U3-1402 in patients with HER3-positive MBC
NCT02980341
U3-1402 in patients with HER3-positive MBC
NCT02980341
U3-1402 in patients with HER3-positive MBC
NCT02980341
IMMU-132 (hRS7-SN38) in patients with epithelial cancers
NCT01631552
IMMU-132 (hRS7-SN38) in patients with epithelial cancers
NCT01631552
IMMU-132 (hRS7-SN38) in patients with epithelial cancers
NCT01631552
ARX788 as a single agent in subjects with advanced cancers with HER2 expression
NCT02512237
First-in-human study of DS-8201A, in subjects with advanced solid malignant tumors
NCT02564900
HSP inhibitors
Ganetespib with paclitaxel, trastuzumab, and pertuzumab in HER2+ MBC
NCT02060253
Bromodomain inhibitors
GSK525762 in subjects with NUT midline carcinoma (NMC) and other cancers
NCT01587703
GSK525762 in subjects with NUT midline carcinoma (NMC) and other cancers
NCT01587703
MK-8628, a small molecule inhibitor of the bromodomain and extra-terminal (BET) proteins, in subjects with selected advanced solid tumors
NCT02698176
Targeting Checkpoints in Breast Cancer: Cell-Cycle and Immune Regulation
Cell-Cycle Regulation: The Role of CDK and Cyclins in Breast Cancer
The transition into each phase of the cell cycle (G1, S, G2, and mitosis) is controlled by checkpoints wherein defects in DNA synthesis are detected [6]. Activation of these checkpoints induces cell-cycle arrest and enables DNA repair. A subset of three interphase cyclin-dependent kinases (CDK2, CDK4, and CDK6), a mitotic CDK (CDK1), and ten cyclins (classified in four groups: A-, B-, D-, and E-type cyclins) form CDK-cyclin complexes that regulate these checkpoints [7], as depicted in Fig. 12.1. Mitogenic stimuli result in expression of D-type cyclins which associate with CDK4 and CDK6, producing hyperphosphorylation and inactivation of the retinoblastoma tumor suppressor protein (Rb) thus allowing transition from G1 to S phase. CDK2-cyclin E complexes further phosphorylate and completely inactivate Rb, resulting in transcription and synthesis of numerous proteins that initiate S phase [8]. Cancer cells may overcome the restriction point in G1 phase through constitutive activation of cyclin D-CDK4/CDK6 or loss of pRb and other inhibitory proteins [9, 10].
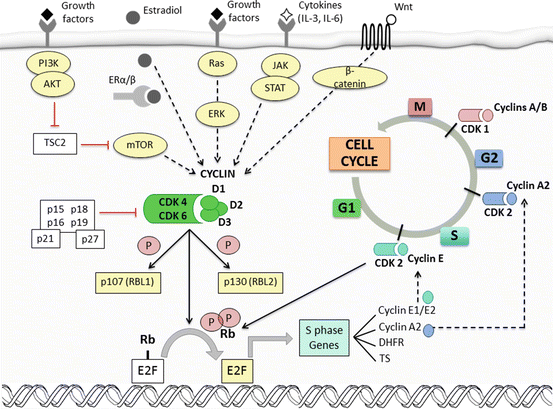

Fig. 12.1
Crosstalk between cell-cycle machinery and oncogenic signaling pathways (Modified with permission from Garrido-Castro A.C., Goel S. Curr Breast Cancer Rep (2017)). The transition into each phase of the cell cycle (G1, S, G2, and mitosis) is tightly controlled by checkpoints that are regulated by CDK-cyclin complexes. Mitogenic stimuli activate intracellular signaling pathways (i.e., PI3K/AKT/mTOR, Ras/Raf/MAPK, JAK/STAT, etc.) that induce the expression of D-type cyclins (D1, D2, and D3). Cyclin D preferentially associates with CDK4 and CDK6, producing hyperphosphorylation and inactivation of retinoblastoma (Rb) by uncoupling it from E2F transcription factors, thus allowing cell-cycle transition from G1 to S phase. In addition, these complexes partially inactivate the inhibitory pocket proteins RBL1 (also known as p107) and RBL2 (also known as p130), enabling the expression of E-type cyclins. CDK2-cyclin E complexes further phosphorylate and completely inactivate these pocket proteins and Rb. This results in transcription and synthesis of numerous proteins that initiate S phase, such as dihydrofolate reductase (DHFR) and thymidylate synthase (TS). A-type cyclins drive the transition from S phase to G2 during later stages of DNA replication and finally activate CDK1 to induce mitosis. After the rupture of the nuclear membrane, degradation of A-type cyclins leads to binding of CDK1 to cyclin B, responsible for entry into mitosis. CDK activation is primarily controlled by binding to cyclins, which show a cyclical pattern of synthesis and degradation. In addition to decreasing levels of D-type cyclins as cells progress through S phase, endogenous inhibition of CDK4/CDK6 is also enabled by two families of CDK inhibitors: the INK4 family (p16INK4A, p15INK4B, p18INK4C, and p19INK4D) and the Cip/Kip family (p21, p27, and p57). Estrogen steroids, such as 17-β-estradiol, promote cell-cycle progression by increasing CCND1 transcription, assembly of active cyclin D1-CDK4 complexes, and, ultimately, pRb phosphorylation. Cyclin D1 can also directly bind to ERα and induce ER-mediated transcription, even in the absence of estradiol
Crosstalk between several oncogenic signaling pathways and cell-cycle machinery has become patent in breast cancer (Fig. 12.1). Cyclin D1 can directly bind to ERα, even in the absence of estradiol, and induce ER-mediated transcription [11]. Cyclin D1 amplification, CDK4 gain, and a greater frequency of alterations of the Rb pathway have been reported in luminal A, luminal B, and HER2-enriched tumors [12]. In contrast to basal subtypes, ER-positive cancer cell lines have been proven the most sensitive to CDK4/CDK6 inhibitors [13]. CDK4/CDK6 inhibitors can also resensitize HER2-amplified tumors that have developed resistance to HER2-directed therapies by reducing TSC2 phosphorylation and attenuating mTOR signaling [5]. Altogether, this evidence supports the rationale for the development of CDK4/CDK6 inhibitors in ER-positive and HER2-positive breast cancer, in combination with endocrine and other targeted therapies.
The current landscape of the treatment of ER-positive breast cancer has rapidly changed with the development of three selective CDK4/CDK6 inhibitors, palbociclib (PD0332991), ribociclib (LEE011), and abemaciclib (LY2835219), each with distinct pharmacokinetic and safety profiles. Abemaciclib has more potent inhibitory activity against CDK4, compared to palbociclib and ribociclib [14], and can also penetrate the central nervous system (CNS) [15]. A greater incidence of fatigue and gastrointestinal disorders has been reported with abemaciclib, compared to higher-grade 3–4 neutropenia observed with the other CDK4/CDK6 inhibitors [16–18]. In terms of efficacy (Table 12.2), palbociclib received US Food and Drug Administration (FDA) accelerated approval in combination with letrozole based on a 10-month progression-free survival (PFS) improvement in postmenopausal women who had no prior therapy for advanced ER-positive disease [19, 20]. Palbociclib was also approved in combination with fulvestrant following progression on prior endocrine therapy, based on data reported from the PALOMA-3 trial [21, 22]. Recently, a randomized placebo-controlled phase III study, MONALEESA-2, met its primary endpoint by demonstrating a significant increase in PFS in patients treated with ribociclib and letrozole in the first-line metastatic setting [23]; at the time of writing, this combination awaits regulatory approval. Abemaciclib was granted FDA Breakthrough Therapy designation after revealing promising antitumoral activity in phase I/II studies, despite inclusion of heavily pretreated patients [18, 24]. Randomized phase III trials of abemaciclib in combination with hormonal therapies in the early-line metastatic setting are ongoing (NCT02107703, NCT02246621).
Table 12.2
Results of phase II/III clinical trials with CDK4/CDK6 inhibitors in advanced breast cancer
CDK inhibitors | |||||
|---|---|---|---|---|---|
Palbociclib | Ribociclib | Abemaciclib | |||
Clinical trial | PALOMA-1 | PALOMA-2 | PALOMA-3 | MONALEESA-2 | MONARCH-1 |
Trial design | Phase II open-label | Phase III double-blind | Phase III double-blind | Phase III double-blind | Phase II single-arm |
Study arms | Palbociclib + letrozole vs. letrozole | Palbociclib + letrozole vs. placebo + letrozole | Palbociclib + fulvestrant vs. placebo + fulvestrant (+/− goserelin) | Ribociclib + letrozole vs. placebo + letrozole | Abemaciclib |
Study drug dose | 125 mg QD 3 w on/1 w off | 125 mg QD 3 w on/1 w off | 125 mg QD 3 w on/1 w off | 600 mg QD 3w on/1w off | 200 mg BID continuously |
Characteristics | |||||
Menopausal status | Postmenopausal | Postmenopausal | All | Postmenopausal | All |
Prior ET allowed for advanced disease | No | No | Yesa | No | Yes |
Total pts, n | 165 | 666 | 521 | 668 | 132 |
Efficacy | |||||
Median PFS (experimental arm vs. control arm), mo | 20.2 vs. 10.2 | 24.8 vs. 14.5 | 9.5 vs. 4.6 | NR vs. 14.7 | 6.0 |
HR PFS (95% CI) | 0.49 (0.32–0.75) | 0.58 (0.46–0.72) | 0.46 (0.36–0.59) | 0.56 (0.43–0.72) | NA |
Median OS (experimental arm vs. control arm), mo | 37.5 vs. 33.3 | NA | NA | NA | 17.7 |
HR OS (95% CI) | 0.81 (0.49–1.35) | NA | NA | NA | NA |
ORR (ITT), % | 43 vs. 33 | 42 vs. 35 | 19 vs. 9 | 41 vs. 28 | 20 |
CBR (ITT), % | 81 vs. 58 | 85 vs. 70 | 67 vs. 40 | 80 vs. 73 | 42.4 |
Safety | |||||
Grade 3–4 neutropenia, % | 54 vs. 1 | 67 vs. 1 | 65 vs. 1 | 59 vs. 1 | 27 |
Grade 3–4 diarrhea, % | 4 vs. 0 | 1 vs. 1 | 0 vs. 1 | 1 vs. 1 | 20 |
Grade 3–4 elevated liver enzymes (ALT; AST), % | 0 vs. 0; 1 vs. 0 | NA | 2 vs. 0; 3 vs. 2 | 9 vs. 1; 6 vs. 1 | NA |
In HER2-positive disease , CDK4/CDK6 inhibitors are currently being explored in phase I/II trials combined with trastuzumab or the antibody-drug conjugate , trastuzumab emtansine (T-DM1), with or without endocrine therapy (NCT02657343, NCT02448420, NCT01976169, NCT02675231). Despite the absence of preclinical data favoring CDK4/CDK6 inhibition in triple-negative breast cancer (TNBC), other CDK targets are under investigation in this subtype. Dinaciclib , a potent inhibitor of CDK1, CDK2, CDK5, and CDK9, has demonstrated encouraging in vitro and in vivo activity [25, 26]. In addition, dinaciclib sensitizes TNBC cell lines to PARP inhibition [27], which encouraged the ongoing phase I trial in combination with veliparib (NCT01434316).
Immune Checkpoint Blockade
The interaction between immunity and cancer has been subject of great interest over the past decades. However, not until recently have immunotherapeutic strategies demonstrated an improvement in patient outcomes in breast cancer. Neoantigens, i.e., peptides arising from cancer specific mutations, are processed by dendritic cells and presented on major histocompatibility class I (MHC-I) and class II (MHC-II) molecules to T cells [28]. The activation of effector T-cell responses against neoantigens requires the establishment of an immunological synapse in which two signals must be present: (1) the interaction between the antigen-MHC complex and the T-cell receptor (TCR) and (2) the presence of the co-stimulatory molecule, CD28 , which binds to its ligands B7-1 (also known as CD80) and B7-2 (CD86) that are expressed by antigen-presenting cells (APC), such as dendritic cells [29]. Both interactions will then stimulate TCR signaling through PI3K-AKT, Ras-Raf-MAPK, and NF-κB pathways, causing secretion of cytokines and promoting proliferation of activated T cells which infiltrate into tumor beds, recognize, and kill cancer cells. However, binding of T-cell inhibitory checkpoint molecules, PD-1 and CTLA-4, to their respective ligands, PD-L1/PD-L2 and CD80/CD86, induces the recruitment of phosphatases that block TCR signaling [28]. Inhibition of these checkpoints has become an attractive strategy, especially for tumors with elevated lymphocytic infiltration.
Traditionally, breast cancer has not been considered highly immunogenic, in part due to the lower prevalence of somatic mutations and less likelihood of formation of neoantigens compared to other tumors, such as melanoma, lung, or urothelial carcinoma [30]. Nevertheless, the presence of tumor-infiltrating lymphocytes (TILs) is prognostic in early-stage triple-negative or HER2-amplified breast cancer [31].
Results from several trials evaluating immune checkpoint inhibitors in the metastatic setting have been reported (Table 12.3) [32–36]. However, comparisons are limited due to broad variations in the inclusion criteria of each study, including differences in tumor subtype, prior number of therapies allowed, preselection based on PD-L1 positivity, and assays used to quantify PD-L1 expression. Common adverse events that have been reported with these agents include arthralgia, fatigue, nausea, diarrhea, and pyrexia. The occurrence of specific immune-related toxicities, such as endocrinopathies, pneumonitis, or hepatitis, is rare and has been attributed to the release of the breaks on autoimmunity. Single-agent checkpoint inhibitors, pembrolizumab and atezolizumab, have achieved overall response rates (ORR) of approximately 19% in heavily pretreated patients with PD-L1-positive TNBC [32, 36]. Conversely, in the JAVELIN trial, ORR in unselected TNBC patients treated with the PD-L1 antibody avelumab was 8.6% and ranged from 6.1% in patients with ≥1% of PD-L1-positive tumor cells to 44.4% in those with ≥10% of PD-L1-positive cells [35], underscoring the need for standardization of assays in the search for predictive biomarkers of response.
Table 12.3
Results of immunotherapy clinical trials in advanced breast cancer
Pembrolizumab in PD-L1+ tumors: TNBC cohort (KEYNOTE-012) | Pembrolizumab in PD-L1+ ER+/HER2− breast cancer (KEYNOTE-028) | Atezolizumab in tumors unselected for PD-L1: TNBC cohort | Atezolizumab + nab-paclitaxel in tumors unselected for PD-L1: TNBC cohort | Avelumab in locally advanced or metastatic breast cancer: unselected for PD-L1 or HR/HER2 status (JAVELIN) | ||||
|---|---|---|---|---|---|---|---|---|
Mechanism of action | Humanized IgG4 anti-PD-1 | Humanized IgG4 Anti-PD-1 | Humanized IgG1 anti-PD-L1 | Humanized IgG1 anti-PD-L1 | Fully human IgG1 anti-PD-L1 | |||
Dose and schedule | 10 mg/kg (Q2W) | 10 mg/kg (Q2W) | 15 mg/kg or 20 mg/kg or 1,200 mg flat dose (Q3W) | Atezolizumab: 800 mg (Q2W); Nab-paclitaxel: 125 mg/kg on days 1,8,15 (Q4W) | 10 mg/kg (Q2W) | |||
Breast cancer subtype | Triple-negative | ER-positive, HER2-negative | Triple-negative | Triple-negative | All | TNBC cohort | HER2-positive cohort | HR-positive, HER2-negative cohort |
PD-L1 positivity definition | ≥1% TC or any staining in stroma | ≥1% TC or any staining in stroma | ≥5% IC | ≥1% IC; ≥1% TC | ≥1% TC; ≥10% IC | ≥1% TC; ≥10% IC | ≥1% TC; ≥10% IC | ≥1% TC; ≥10% IC |
PD-L1 inclusion criteria | Positive | Positive | All-comersa | All-comers | All-comers | |||
No. PD-L1+ pts/no. PD-L1 evaluable cases (%) | 65/111 (58.6) | 48/248 (19.4) | 37/54 (68.5) | 9/24 (37.5); 3/24 (12.5) | 85/136 (62.5); 12/136 (8.8) | 33/48 (68.8); 9/48 (18.8) | 15/21 (71.4); 1/21 (4.8) | 31/56 (55.4); 2/56 (3.6) |
No. pts enrolled | 32 | 25 | 54 | 32 | 168 | 58 | 26 | 72 |
No. pts included in efficacy analysis | 27 | 25 | 21a | 24 | 168 | 58 | 26 | 72 |
No. prior therapies for metastatic disease, median (range) | 2 (0–9) | NA | NA | 5 (1–10) | 3 (0–10) | NA | NA | NA |
No. pts with ≥3 prior therapies for metastatic disease (%) | 15 (46.9) | 20 (80.0) | NAb | 1 (4.2) | 88 (52.4) | 13 (22.4) | NA | NA |
ORR, % | 18.5 | 12.0c | 19.0 | 70.8d | 4.8 | 8.6 | 3.8 | 2.8 |
ORR in PD-L1+ cohort, % | 18.5 | 12.0c | 19.0 | 77.8 | 3.5; 33.3 | 6.1; 44.4 | NA | NA |
CBR, % | 25.9 | 20.0c | NA | NA | 28.0 | 31.0 | NA | NA |
Median DOR, wks (range) | NR (15.0 to 47.3+) | NR (8.7+ to 44.3+) | NR (18 to 56+) | NA
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

| ||||