- Continuous subcutaneous insulin infusion pumps and continuous glucose monitors can be effective tools for improving metabolic control in patients with type 1 diabetes.
- Pump therapy provides increased lifestyle flexibility, and can reduce the risk for hypoglycemia.
- Real-time continuous glucose monitoring devices provide detailed information on glucose patterns and trends, and alarms that are triggered by both hyperglycemia and hypoglycemia.
- Randomized trials indicate that this technology can lead to a reduction in HbA1c without an associated increase in hypoglycemia.
Introduction
The development of capillary blood glucose monitors set the stage for the era of intensive insulin therapy, and technologic advances continue to be an important driver for improvements in diabetes care. In this chapter we highlight the developments of the past decade focusing on those technologies that show the most promise in improving the lives of people with diabetes, in particular, insulin pump therapy and real-time continuous glucose monitoring. In recent years, inhaled insulin has been an area of intense investigation; however, interest in this mode of insulin delivery has been diminished by concerns about potential pulmonary toxicity.
Continuous subcutaneous insulin infusion pumps
Continuous subcutaneous insulin infusion (CSII) pumps were first introduced over 30 years ago [1,2]. They consist of an insulin reservoir and a delivery catheter that continuously infuses insulin into the subcutaneous tissue. In recent years, there has been growing adoption of this technology in diabetes care. Several factors have contributed to this increased use, including the focus on intensive therapy triggered by the Diabetes Control and Complications Trial (DCCT); improvements in the reliability and usability of pumps; patient preferences; and also the growing recognition by clinicians that CSII delivery is associated with reduced risk for hypoglycemia. Because a comprehensive review of insulin pump therapy is beyond the scope of this chapter, we focus on issues that have received most attention in the recent literature, including the potential advantages of pumps as a tool for insulin administration and newer developments in pump technology such as bolus calculator software. In addition, we provide some practical pointers about pump therapy for the clinician.
Glycemic control with insulin pumps compared with multiple daily insulin injections
Several published meta-analyses have examined the role of CSII pumps as a tool for intensifying glycemic control [3–5]. These meta-analyses of the randomized controlled trials in the published literature indicate that CSII use in patients with type 1 diabetes mellitus (T1DM) is associated with 0.4–0.5% (5–6 mmol/mol) lower HbAlc than multiple daily injection (MDI) therapy. The improvements in HbA1c with the change to pump therapy appear to be greater in individuals who have poorer glycemic control [6,7]. Most of the studies examined in these meta-analyses involved MDI regimens using NPH or ultralente insulins; however, more recent randomized controlled trials suggest that CSII is also superior to MDI regimens using the newer long-acting insulin analogs [8,9]. Data about potential complications of pump therapy (such as ketoacidosis, pump malfunction and insulin site problems) is not reported in many of the published randomized trials, and most evidence suggesting that CSII is associated with an increased risk for ketoacidosis date from the 1980s [10–12]; however, device malfunction and infusion site problems do still occur with modern pump technology, which points to the importance of giving education on proper catheter site care and ketoacidosis prevention to all patients using pumps.
Because of the more controlled delivery of insulin, CSII can be an effective tool for reducing glycemic variability, and this can be of benefit for reducing the risk for hypoglycemia brought about by insulin replacement therapy [13]. Clinical guidelines from several national and international organizations recommend consideration of pump therapy for individuals with T1DM with suboptimal glycemic control and problematic hypoglycemia (Box 28.1) [14–16]. However, conclusions from the meta-analyses about whether the mode of insulin delivery has an impact on hypoglycemia have yielded conflicting results [5,17,18], in large part because of methodologic issues and differences in trial selection [19,20]. The meta-analysis of randomized controlled trials by Pickup & Sutton [17], which was restricted to studies published since 1995 (i.e. since the introduction of more reliable pump technology) with a baseline (pre-trial) rate of severe hypoglycemia of >10 episodes/100 patient years and ≥6 months of CSII use, showed that there was a 2.9-fold (95% confidence interval [CI] 1.45–5.76) reduction in severe hypoglycemia during CSII compared to MDI. The analysis also indicated that the benefit from pump therapy was greater in individuals with higher rates of severe hypoglycemia (P < 0.001) and those with longer duration of diabetes (P = 0.025) [17]. A meta-analysis commissioned by the Endocrine Society reached different findings, and concluded that in comparison with MDI, CSII was not associated with a significant reduction in severe or nocturnal hypoglycemia [18]. The validity of these conclusions is limited by the inclusion of studies of relatively short duration with low incidence rates of severe hypoglycemia that would bias against the detection of any potential benefit from pump therapy. In addition, the rates of minor and nocturnal hypoglycemia were determined using intermittent fingerstick glucose monitoring, which can be unreliable in detecting nocturnal hypoglycemic events [21] and would therefore be relatively insensitive to detecting treatment-related differences. Furthermore, it should also be noted that the studies examined in this analysis were almost entirely performed using older pump types that did not incorporate the bolus calculator software now available in updated pumps which can help to limit hypoglycemia related to doses stacking up from repeated boluses. These factors limit the generalizability of the conclusions from this analysis to clinical practice, and the weight of evidence from studies reported in the literature suggest that clinicians caring for motivated patients with T1DM who have recurrent hypoglycemia and especially a history of severe hypoglycemic reactions should recommend a trial of insulin pump therapy.
Evidence in insulin-requiring type 2 diabetes mellitus (T2DM) regarding the potential benefits of pump therapy in comparison with MDI is limited. The studies of Raskin et al. [22] and Herman et al. [23] showed similar HbA1c changes with both modes of insulin delivery. A small-scale cross-over study in obese subjects with T2DM with insulin resistance has demonstrated a significant reduction in HbA1c and postmeal glucose excursion with use of pump therapy [24]. Where patients are unable to achieve adequate glycemic control, several practical issues should be considered (Box 28.2).
- Severe hypoglycemia and hypoglycemia unawareness
- HbA1c above goal
- Caution: Intentional insulin omission (to facilitate weight loss) is a not uncommon cause for poorly controlled diabetes, especially in young women [32]. Before initiating pump therapy, it is important to rule out this problem. Because these patients habitually underdose insulin and are frequently hyperglycemic, they do not routinely troubleshoot for insulin non-delivery by the pump and can therefore be at increased risk for developing ketoacidosis
- Diurnal variations in basal insulin requirements caused by the dawn phenomenon [33,34] and steroid therapy can be more readily managed using the multiple basal rates provided by the pump than by long-acting injected insulins [35]. Continuous subcutaneous insulin infusion can be of special benefit for the post-renal transplant diabetes patient on steroid therapy who is striving for intensive glycemic control
- Preconception and pregnancy
- Practical advantages of pumps for bolus insulin delivery include:
- Dosing precision: The extra precision of insulin dosing with pumps can be an important advantage for young children (especially infants and neonates) [36] and adults who are on very low insulin doses. In addition, accurate dosing of insulin boluses in fractions of a unit allows the patient to correct hyperglycemia more precisely without overshooting and causing hypoglycemia. For those patients in whom fear of hypoglycemia is an impediment to tight glycemic control, this added assurance can be critical in overcoming reluctance to intensification. In practice, it can be helpful to reduce missed food boluses, facilitate interprandial “correction” bolusing, and help simplify eating at restaurants and social occasions (with the use of extended/square wave boluses and multiple bolusing)
- Optimizing post-prandial insulin coverage: Facilitates dosing for higher fat, complex carbohydrate and/or larger meals. Dietary fat delays gastric emptying [37] and induces postprandial insulin resistance [38], so high-fat meals cannot usually be adequately covered using a single injection of rapid-acting insulin [39]. Use of the extended/dual bolus and increased temporary basal can help optimize post-prandial glycemic control following these meals [40–42]. (For practical strategies see Wolpert [43].)
- Gastroparesis: Use of the extended/dual wave bolus can allow for better matching of carbohydrate absorption and insulin action
- Infusion site issues including scarring, lipohypertrophy and lipoatrophy: Infusion sites should be routinely examined in pump users with unexplained glucose fluctuations
- Infusion catheter kinking or dislodgement: Plastic catheters that are perpendicular to the skin surface and have a small base for attachment (e.g. Medtronic MiniMed Sofset™, Disetronic Ultraflex™) are more prone to kink or become dislodged especially with activity or perspiration. Solutions include changing to metal needle infusion sets, plastic sets with a shorter cannula, or other types of plastic infusion sets that are less prone to kinking. These include the Medtronic MiniMed Quickset™ (which has a broad base of attachment to the skin), and sets that insert obliquely such as the Medtronic MiniMed Silouette™, Roche Disetronic Tender™ and Animas Comfort™
- Failure to change the pump reservoir and infusion system on a regular basis: This may manifest in a tendency for elevated and erratic glucose in the period preceding infusion set changes
- Review of pump downloads can be helpful:
- Check priming history to assess how frequently the infusion system is being changed
- Check bolus history to detect possible missed meal boluses
- Check percentage of basal to bolus insulin. A high percentage of basal insulin in the patient with frequent hyperglycemia may indicate that bolus doses are frequently being missed. A high percentage of basal insulin in the patient with frequent hypoglycemia may indicate that high basal rates are contributing to hypoglycemia, and would point to a need to re-evaluate basal rate settings
- Check for a history of pump suspension or basal rate reduction. Even temporary removal of the pump to bathe can lead to elevations in the glucose levels; patients need to be reminded to bolus to replace the missed basal when reconnecting the pump. Some patients using the pump will get in the practice of reducing the basal rate or suspending the pump when they are hypoglycemic; the end result will often be exaggerated rebound hyperglycemia
- Insulin instability in the pump infusion system: This can manifest as increases in the glucose levels in the period preceding infusion set changes or even precipitation in the infusion system [46]
- Pump malfunction
- Air bubbles in infusion system resulting from poor filling technique
Quality of life benefits and patient expectations
Many patients describe improvements in quality of life when they change to pump therapy; however, there have been few carefully designed studies that have examined patient perspectives of pump therapy, and differences in psychosocial functioning with CSII compared to MDI [4,25]. The published literature suggests that use of pumps is associated with improved quality of life compared to MDI [26–28]; however, this has not been confirmed in all studies [29]. Patients will vary in their perceptions about the potential quality of life benefits (including increased lifestyle flexibility, dietary freedom, reduced fear for hypoglycemia) relative to some of the potential drawbacks of CSII (including body image concerns and the need for frequent blood glucose monitoring) [30]. Wolpert and colleagues conducted a focus group investigation of 30 patients followed at the Joslin Diabetes Center to examine how psychosocial factors impacted the use of the pump [31]. Patients with better glycemic control viewed the pump as a tool for diabetes self-management rather than as a panacea. In contrast, the pump patients with poorer HbA1c had more unrealistic expectations including the perception that use of technology was a substitute for attentiveness to self-care and that pump therapy allowed them to do whatever they wanted, particularly with regard to eating. Before initiating pump therapy, health care professionals need to assess patients’ expectations of the pump, dispel unrealistic notions (often promoted by marketing materials) and ensure that patients recognize that while the pump allows life with diabetes to be more flexible and convenient, it is not a vehicle for total freedom from diabetes.
New developments in pump technology: bolus calculators
In recent years, new software programs that assist patients with bolus calculations have been incorporated into insulin pumps. The patient’s insulin: carbohydrate ratio (the number of carbohydrate grams covered by 1 unit of insulin) and correction/sensitivity factor (a measure of the glucose-lowering effect of 1 unit of insulin) is programmed into the pump software. Based on planned carbohydrate intake of the patient and the blood glucose level, the bolus calculator will recommend a bolus dose. Although this dose calculation software can simplify the daily self-care routines of the pump users, patients should receive appropriate education to ensure that they can manually calculate bolus doses in the event they need to discontinue pump therapy. In addition, it is important that the programming of the bolus calculator software is adjusted to individual needs and not automatically left at the manufacturer’s default settings. These bolus calculators have an important role in minimizing the risk for hypoglycemia from multiple boluses and dose stacking which are a common practical problem with some pump users [43]. When calculating an insulin dose, the pump software will consider the amount of active insulin-on-board (commonly referred to as IOB) and subtract this IOB from the recommended dose. This IOB calculation is based on insulin action plots which predict the amount of insulin remaining as a function of time; the assumed insulin duration of action used by the software in this calculation is individually programmed by the patient or health care professional, and can be varied from 2 to 8 hours depending on the pump type [44]. As outlined in Chapter 27 the absorption of insulin analogs can vary markedly even within individuals. In practice, the major consideration in setting the insulin duration of action in the bolus calculator software is usually a best guess based on a clinical assessment of the hypoglycemia risk of the patient and the imperative for achieving tight glycemic control (in particular, preconception and pregnancy) [45]. For individuals with frequent hypoglycemia, hypoglycemia unawareness or a history of severe hypoglycemic reactions, the duration of action should be set 5 hours or even longer, whereas for the woman who is pregnant or trying to conceive, the duration of action is often set at 2–3 hours. The glucose records of the patient can be assessed to determine if the duration of action settings are appropriate. If the duration of action time programmed into the software is less than the true duration of action, the pump will indicate that there is less IOB than is actually the case, and the patient will mistakenly take more insulin than required; the glucose records will show evidence of hypoglycemia from “stacking” of doses. Conversely, if the setting programmed into the pump is longer than the true duration of insulin action, this can lead to underdosing of insulin and hinder attempts to optimize glycemic control.
Real-time continuous glucose monitoring
In the last several years, several real-time continuous glucose monitoring (RT-CGM) devices (Abbott Freestyle Navigator®, DexCom Seven®, Medtronic Guardian®/Paradigm® system) have received the CE mark and approval from the US Food and Drug Administration (FDA) for long-term use in ambulatory patients with diabetes (Figure 28.1). These devices have transcutaneous glucose oxidase-based electrochemical sensors that measure the glucose concentration in the interstitial fluid every 1–5 minutes (Figure 28.2). Because of accuracy limitations [47,48], the current generation of CGM devices do not have regulatory approval for use as stand-alone devices, and patients are required to perform adjunctive capillary blood glucose measurements; however, this limitation is compensated by the additional detailed information provided by the CGM about glucose patterns, the rate and direction of change in the glucose level and adjustable alarms that are triggered by both hyperglycemia and hypoglycemia (Figure 28.3) [49].
Figure 28.1 Three components of continuous glucose monitoring (CGM) device: sensor, transmitter and receiver unit.
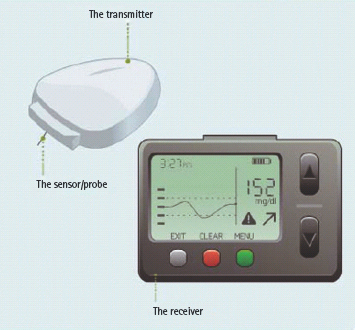
Figure 28.2 Modal day report from patient who eats breakfast at 9:00 am and supper at 10:00 pm daily. Indicates rapid spike in glucose levels post-meal that had not been detected with intermittent fingerstick blood glucose monitoring. Treatment recommendations focused on changing to more slowly absorbed lower glycemic index carbohydrates (e.g. oatmeal) and/or injection of bolus insulin earlier before the meal.
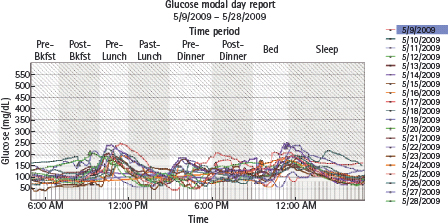
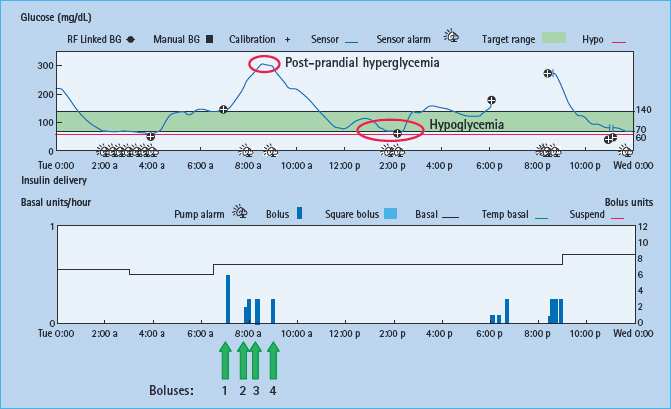
Figure 28.3 Device download: top panel shows continuous glucose tracing, and bottom panel shows insulin delivery (each blue bar represents an insulin bolus with units of insulin shown on the vertical axis on the right). Following breakfast the glucose level increased to 300 mg/dL (16.7 mmol/L) prompting the patient to take multiple boluses with resultant hypoglycemia.
Clinical efficacy of RT-CGM
Because RT-CGM is a relatively new technology, to date only a few randomized controlled trials have been performed. The initial reported studies, which were only several days in duration, showed that use of RT-CGM leads to a reduction of both hyperglycemic and hypoglycemic excursions with more time in the target glucose range [50,51]. The Guard Control trial using the Medtronic Guardian was the first longer duration study of RT-CGM. This study enrolled 156 adults and children with T1DM using both pumps and MDI therapy [52]. At 3-month follow-up, the group randomized to RT-CGM use had 1.0 ± 1.1% (11 ± 12mmol/mol) reduction in HbA1c (down from 9.5 ± 1.1% [80 ± 12 mmol/mol] at baseline) compared to 0.4 ± 1.0% (5 ± 11 mmol/mol) reduction in the control group (P = 0.003). In addition, at 3 months, 50% of subjects in the CGM group had HbA1c reductions of ≥1% compared to 15% of subjects in the control group, and 26% in the CGM group had HbA1c reductions of ≥2% compared to 4% in the control arm. Reported results from this industry-supported study have not included any specific information on the treatment protocols used in the trial or data about the effect of CGM on hypoglycemia.
To date, the results of two large-scale multicenter randomized controlled trials of 6 months’ duration have been published in the literature: the industry-supported Star 1 trial and the Juvenile Diabetes Research Foundation (JDRF) sponsored CGM trial. The Star 1 trial enrolled 146 subjects aged 12–72 years with T1DM treated with CSII pumps [53] who were randomized to pump therapy with RT-CGM using the Medtronic 722 pump system or pump therapy with fingerstick self-monitoring of blood glucose. The primary endpoint was not achieved; there was a similar reduction in HbA1c (0.71 ± 0.71% in the sensor group vs 0.56 ± 0.072% in the control group; P = non-significant) over the 6-month trial. Importantly, in the subjects using RT-CGM, the improvement in glycemic control was accomplished without any change in biochemical hypoglycemia (P < 0.0002), whereas in the control subjects using fingerstick glucose monitoring there was an increase in biochemical hypoglycemia (documented with blinded CGM devices). More severe hypoglycemic events occurred in the group using RT-CGM than in the control group (11 vs 4; P = 0.04). During six of the 11 severe events in the RT-CGM group, subjects were not using a sensor. The Data Safety Monitoring Board determined that in the remaining five severe hypoglycemic events there was evidence of the following:
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree