Sickle cell disease is a common, inherited disordered characterized by chronic hemolytic anemia with repetitive episodes of vasoocclusion resulting from deformed red blood cells. This article reviews the most significant neurologic and head and neck manifestations of this disease.
Key points
- •
Clinical symptoms of sickle cell disease (SCD) occur primarily as a result of hemolytic anemia, vasocclusion, infection, or a combination thereof.
- •
Hemorrhagic and ischemic stroke is the leading cause of death and a major cause of morbidity in SCD patients.
- •
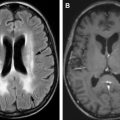
SCD results in diffuse gray and white matter abnormalities, which may account for accelerated neurocognitive deficits even in patients without focal lesions on conventional MRI.
- •
SCD-associated vasculopathy may result in vascular narrowing or aneurysm formation. Chronic progressive occlusion of the internal carotid artery with prominent lenticulostriate collaterals produces the stereotypical “moyamoya” angiographic pattern.
- •
Although less well-known, many patients suffer from head and neck manifestations of SCD, which may affect the inner ears, orbits, sinuses, lymphoid tissue, and bone.
Background
Sickle cell disease (SCD) is an autosomal-recessive inherited disorder characterized by an abnormal oxygen-carrying hemoglobin molecule that results in deformed red blood cells (RBC). This widely studied entity was the first disease where the exact genetic and molecular defect was identified, attributable to a single nucleotide mutation of the β-globin gene located on the short arm of chromosome 11. The disease requires 2 defective genes for full disease penetrance and most patients are homozygotic HbS carriers. Although the term SCD refers to a somewhat varied population with clinically important compound heterozygous variants, including sickle D, sickle C, and sickle B thalassemia.
SCD is common, with approximately 300,000 children born annually with the disease, predominating in sub-Saharan African and among African descendants around the world. The Centers for Disease Control and Prevention estimates that 90,000 to 100,000 Americans suffer from the disease. Despite recent advances in treatment, the high morbidity and mortality rates of SCD remain a major public health concern resulting in an average of 75,000 hospitalizations and US health care costs approaching $475 million annually.
Low oxygen tension results in polymerization of the abnormal hemoglobin tetramer, which becomes relative insoluble when deoxygenated. Aggregates form long chains, disrupting the microstructural and macrostructural appearance of the cell. The normally plastic biconcave erythrocytes morph into crescentic or sickle-shaped cells that are more friable and easily hemolyzed. Preceding events may include infection, trauma, and other stressful conditions that may promote intracellular hypoxia and acidosis.
Acute and chronic clinical manifestations of SCD primarily occur as a result of hemolysis and vasoocclusion. Changes in membrane structure and function, loss of normal plasticity, disorganized cell volume control, increased sheer stress, and increased endothelial adherence are all thought to contribute to these phenomena. A typical RBC from a sickle cell patient has a life span on the order of just 10 to 20 days in comparison with the normal 90 to 120 days. Chronic anemia results in profound stresses on both the hematopoietic and cardiovascular systems.
After RBC sickling, vasoocclusion occurs through a cascade of events including endothelial adhesion and damage, arterial narrowing, and aggregation, ultimately leading to ischemia and/or infarction. Ischemic events may occur throughout the body, resulting in repeated acutely painful episodes known as crises. Hemolysis and vasoocclusion result in end organ damage affecting virtually every organ system to some degree. This review focuses on the central nervous system and the less recognized head and neck manifestations of the disease.
Background
Sickle cell disease (SCD) is an autosomal-recessive inherited disorder characterized by an abnormal oxygen-carrying hemoglobin molecule that results in deformed red blood cells (RBC). This widely studied entity was the first disease where the exact genetic and molecular defect was identified, attributable to a single nucleotide mutation of the β-globin gene located on the short arm of chromosome 11. The disease requires 2 defective genes for full disease penetrance and most patients are homozygotic HbS carriers. Although the term SCD refers to a somewhat varied population with clinically important compound heterozygous variants, including sickle D, sickle C, and sickle B thalassemia.
SCD is common, with approximately 300,000 children born annually with the disease, predominating in sub-Saharan African and among African descendants around the world. The Centers for Disease Control and Prevention estimates that 90,000 to 100,000 Americans suffer from the disease. Despite recent advances in treatment, the high morbidity and mortality rates of SCD remain a major public health concern resulting in an average of 75,000 hospitalizations and US health care costs approaching $475 million annually.
Low oxygen tension results in polymerization of the abnormal hemoglobin tetramer, which becomes relative insoluble when deoxygenated. Aggregates form long chains, disrupting the microstructural and macrostructural appearance of the cell. The normally plastic biconcave erythrocytes morph into crescentic or sickle-shaped cells that are more friable and easily hemolyzed. Preceding events may include infection, trauma, and other stressful conditions that may promote intracellular hypoxia and acidosis.
Acute and chronic clinical manifestations of SCD primarily occur as a result of hemolysis and vasoocclusion. Changes in membrane structure and function, loss of normal plasticity, disorganized cell volume control, increased sheer stress, and increased endothelial adherence are all thought to contribute to these phenomena. A typical RBC from a sickle cell patient has a life span on the order of just 10 to 20 days in comparison with the normal 90 to 120 days. Chronic anemia results in profound stresses on both the hematopoietic and cardiovascular systems.
After RBC sickling, vasoocclusion occurs through a cascade of events including endothelial adhesion and damage, arterial narrowing, and aggregation, ultimately leading to ischemia and/or infarction. Ischemic events may occur throughout the body, resulting in repeated acutely painful episodes known as crises. Hemolysis and vasoocclusion result in end organ damage affecting virtually every organ system to some degree. This review focuses on the central nervous system and the less recognized head and neck manifestations of the disease.
Vascular disease
SCD vasculopathy results in large vessel arterial stenosis and occlusion with the distal internal carotid arteries, proximal anterior cerebral arteries, and middle cerebral arteries (MCA) most commonly affected ( Fig. 1 ). Pathologic studies describe smooth muscle hyperplasia and intraluminal thrombus rather than inflammation or atherosclerosis as the underlying pathophysiology. This process occurs slowly over time as evidenced by extensive collateral vessel formation, particularly the lenticulostriate vessels coursing through the basal ganglia. The angiographic appearance of stenosis with an extensive network of ill-defined collateral vessels was likened to “a puff of smoke” by the original Japanese angiographers who coined the term “moyamoya” ( Fig. 2 ). This moyamoya pattern has been described in several other disease entities including neurofibromatosis and postradiation vasculopathy.
Blood supply is tenuous, even in the setting of collateral vessel formation with regional perfusion abnormalities. These collateral vessels exhibit stress-related changes, including thinned walls and microaneurysm formation, predisposing them to both hemorrhage and thrombosis.
In addition to stenosis, SCD may result in arterial dilatation. Reported rates of aneurysm formation in SCD patients appear much higher than that of the general population (>1% in children and 10% in adults), with a high prevalence of patients with multiple aneurysms. Chronic high-flow states, changes in circulatory patterns, and underlying vessel wall damage all likely contribute to the increased rates of aneurysms. Nonsaccular arterial enlargement (or ectasia) has also been described in the setting of SCD, most commonly affecting the basilar artery, but also seen within the anterior circulation as well ( Fig. 3 ).
Transcranial Doppler ultrasonography may be used to assess the blood flow within internal carotid arteries and MCA. The velocity of flowing blood is inversely proportional to arterial diameter; therefore, stenosis may be identified by localizing areas of increased velocity and high-resistance waveforms. Absent waveforms suggest complete occlusion. Although there are significant limitations related to specificity and anatomic accuracy, the technique is helpful in identifying patients with stenoses and those at risk for stroke.
Computed tomography (CT) and MR angiography are excellent noninvasive imaging techniques to identify areas of vascular narrowing. Each technique has its own strengths and limitations; however, both focus on evaluation of flowing blood within the arterial lumen. CT angiography offers improved spatial resolution and is less susceptible to artifact. However, MR angiography is generally recommended in patients who are expected to undergo repeated examinations and routine follow-up to minimize radiation exposure.
Although dedicated evaluation of the vasculature is not included in a typical MRI of the brain, subtle findings may indicate the presence of stenosis and collateral formation, even in the absence of ischemic changes. Loss of the normal internal carotid arterial flow voids with punctate basal ganglia flow voids on T2-weighted images are highly suspicious. Tiny foci of postcontrast enhancement within the basal ganglia or an ill-defined enhancing structure replacing the normal MCA in the Sylvain fissure indicates collateral vessel formation ( Fig. 4 ). The “ivy sign” refers to leptomeningeal enhancement in the cerebral sulci indicating engorged pial collateral vessels.
Aneurysms should be readily identifiable on CT or MR angiography as focal saccular outpouchings. These tend to occur at predictable locations—vascular branch points including the anterior communication artery, posterior communicating artery, and middle cerebral artery bifurcation. However, the typical search pattern should be expanded to avoid missing aneurysm in atypical locations, especially including the posterior circulation.
Neurologic complications
Infarction
Stroke is a major complication associated with SCD, and the leading cause of death in both children and adults with the disease. CDC estimates a 300-fold increased likelihood of stroke for children with SCD, with an age-adjusted incidence is 0.61 to 0.76 per 100 patient-years. Cerebral infarction affects approximately 30% of all individuals with SCD. Normal brain function requires a continuous supply of oxygen to maintain aerobic metabolism. Ischemic infarctions occur as a result of both occlusive disease and hemodynamic factors, including a reduction in arterial oxygen concentration. Clinically, a stroke is manifested as an acute onset focal neurologic deficit lasting for more than 24 hours. Symptoms vary with the site and size of the lesion, but may include motor or sensory deficits, vision or hearing loss, or disruption of language function.
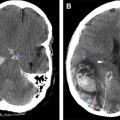
CT serves as the initial study of choice for acutely symptomatic stroke patients owing to its widespread availability and speed of imaging. It accurately distinguishes hemorrhagic from ischemic lesions, facilitating triage and time-sensitive treatments such as intravenous or intraarterial thrombolytics. Classic CT findings in a large vessel acute ischemic infarction include decreased attenuation with loss of gray–white differentiation conforming to a specific vascular distribution. CT findings of ischemic stroke may take hours to develop, so a normal appearing scan does not exclude the diagnosis. The presence of a hyperattenuating vessel, the so-called hyperdense MCA sign, is another finding that may indicate a thrombus in a vessel. Mass effect from infarction develops in the first few days and wanes in the subacute to chronic phases ( Fig. 5 ).
MRI is an excellent modality to assess acute, subacute, and chronic ischemic changes. After a screening CT examination, diffusion-weighted imaging has become the standard of care for MR evaluation of stroke in the last few decades. Cytotoxic edema from infarction restricts the free diffusivity of water molecules resulting in increased diffusion-weighted imaging signal and decreased signal on apparent diffusion coefficient maps. In contradistinction to CT, diffusion-weighted imaging may identify infarcted brain tissue in a matter of minutes ( Fig. 6 ). Signal changes on T2-weighted images and fluid-attenuated inversion recovery develop in the first 6 to 12 hours, and commonly persist. Gliosis and encephalomalacia from chronic infarcts manifest as focal volume loss with increased signal on T2-weighted images ( Fig. 7 ).

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree