Leukemias are a heterogeneous group of hematologic malignancies that results from uncontrolled neoplastic proliferation of undifferentiated or partially differentiated hematopoietic cells. Patients with acute leukemia can have a variety of craniocerebral complications, which can result from direct leukemic involvement, secondary to cerebrovascular or infectious complications of leukemia, or can be treatment related. Imaging plays a central role in evaluating the central nervous system during treatment in patients with leukemia. CT scan is usually considered an effective initial imaging modality to evaluate for cerebrovascular complications. MRI is considered the imaging modality of choice due to its versatility.
Key points
- •
Localized collection of leukemic cells can present as mass lesions, which can involve multiple organs, including the central nervous system and head and neck, and are called granulocytic sarcoma.
- •
Granulocytic sarcomas are usually hyperdense on computed tomographic scans due to cellularity and may present as multiple masses.
- •
On MRI, granulocytic sarcomas are usually isointense to gray matter on T1-weighted sequences and demonstrate homogeneous contrast enhancement. Diffusion restricted may be also seen, indicative of cellularity.
- •
A variety of cerebrovascular complications can occur in patients with leukemia, including intraparenchymal hemorrhage, leukostasis, and cerebral venous infarctions.
Introduction
Leukemias are a heterogeneous group of hematologic malignancies that results from uncontrolled neoplastic proliferation of undifferentiated or partially differentiated hematopoietic cells. Patients with acute leukemia can have a variety of craniocerebral complications, which can result from direct leukemic involvement, secondary to cerebrovascular or infectious complications of leukemia, or can be treatment related.
Leukemia is the commonest childhood cancer, accounting for one-fourth to one-third of all childhood malignancy patients. Most cases (75%) of acute leukemia in the pediatric population are acute lymphoblastic leukemia (ALL), with acute myeloid leukemia (AML) accounting for only 20% of cases. Chronic myelogenous leukemia (CML), juvenile myelomonocytic leukemia (JMML), and myelodysplastic myeloproliferative syndrome account for approximately 5% of childhood leukemias. ALL accounts for approximately 15% to 20% of all adult acute leukemias. Central nervous system (CNS) involvement is identified at the time of diagnosis in less than 10% of adult ALL and is not an independent poor prognostic factor; however, leukemic relapse remains a major therapeutic problem, and CNS involvement at the time of relapse, which occurs in 1% to 15% of cases, is associated with poor prognosis. CNS involvement in adults with AML is less common when compared with ALL. In a study of 395 patients with AML, only 1.8% of patients had initial CNS involvement and 1% suffered an isolated CNS relapse. Several risk factors have been associated with development of CNS leukemia, including age (young adult), mature B-cell ALL, and T-cell leukemia.
Although there has been an increase in cure rates of patients with leukemia, secondary to emergence of new treatment modalities, including aggressive chemotherapy, intrathecal prophylaxis, cranial irradiation, and bone marrow transplantation, use of these intensive treatment regimens have also led to new adverse consequences, including neurotoxicity secondary to various chemotherapeutic agents, acute and late effects of CNS radiation, coagulopathy, immunosuppression, and bone marrow suppression.
Introduction
Leukemias are a heterogeneous group of hematologic malignancies that results from uncontrolled neoplastic proliferation of undifferentiated or partially differentiated hematopoietic cells. Patients with acute leukemia can have a variety of craniocerebral complications, which can result from direct leukemic involvement, secondary to cerebrovascular or infectious complications of leukemia, or can be treatment related.
Leukemia is the commonest childhood cancer, accounting for one-fourth to one-third of all childhood malignancy patients. Most cases (75%) of acute leukemia in the pediatric population are acute lymphoblastic leukemia (ALL), with acute myeloid leukemia (AML) accounting for only 20% of cases. Chronic myelogenous leukemia (CML), juvenile myelomonocytic leukemia (JMML), and myelodysplastic myeloproliferative syndrome account for approximately 5% of childhood leukemias. ALL accounts for approximately 15% to 20% of all adult acute leukemias. Central nervous system (CNS) involvement is identified at the time of diagnosis in less than 10% of adult ALL and is not an independent poor prognostic factor; however, leukemic relapse remains a major therapeutic problem, and CNS involvement at the time of relapse, which occurs in 1% to 15% of cases, is associated with poor prognosis. CNS involvement in adults with AML is less common when compared with ALL. In a study of 395 patients with AML, only 1.8% of patients had initial CNS involvement and 1% suffered an isolated CNS relapse. Several risk factors have been associated with development of CNS leukemia, including age (young adult), mature B-cell ALL, and T-cell leukemia.
Although there has been an increase in cure rates of patients with leukemia, secondary to emergence of new treatment modalities, including aggressive chemotherapy, intrathecal prophylaxis, cranial irradiation, and bone marrow transplantation, use of these intensive treatment regimens have also led to new adverse consequences, including neurotoxicity secondary to various chemotherapeutic agents, acute and late effects of CNS radiation, coagulopathy, immunosuppression, and bone marrow suppression.
Direct leukemic involvement of central nervous system
In leukemia, localized collection of leukemic cells can present as mass lesions, which can involve multiple organs, including the CNS and head and neck. It is more commonly encountered in patients with myeloid leukemia and has been historically called chloroma secondary to greenish hue of the mass on gross specimen secondary to the presence of myeloperoxidase; however, granulocytic sarcoma is considered the preferred terminology because not all lesions are green.
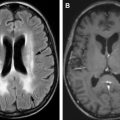
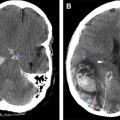
Within the head and neck, granulocytic sarcoma can involve skin and subcutaneous tissues, lymph nodes, and calvarium. Additional sites of involvement include orbits, paranasal sinuses, nasopharynx, and temporal bone. In addition, they can involve the intracranial structures including the dura ( Figs. 1 and 2 ), with occasional indentation of the subarachnoid space and secondary growth into the brain parenchyma through the perivascular spaces. Granulocytic sarcomas are usually hyperdense on computed tomographic (CT) scan, which results from dense cellularity and may present as single or multiple masses. On MRI, restricted diffusion may be seen, also indicative of cellularity (see Fig. 1 B, C and Fig. 2 D, E). Lesions are usually isointense to gray matter on T1-weighted sequences and demonstrate relatively homogeneous enhancement following contrast administration (see Fig. 1 D). Thus, imaging features are similar to lymphoma. Homogeneous enhancement argues for lymphoma or leukemia and against other enhancing neoplasms, such as solid organ metastases or glioblastoma, which typically show cystic changes or necrosis. Advanced imaging techniques can improve diagnostic certainty. Magnetic resonance spectroscopy demonstrates a neoplastic spectrum with elevated choline indicative of increased cellularity and cell membrane turnover (see Fig. 1 E). In contrast to metastases and glioblastoma, leukemia (and lymphoma) masses show little elevation of relative cerebral blood volume (see Fig. 1 F). The differential diagnosis for intraparenchymal granulocytic sarcoma is hemorrhagic infarct, which can result from coagulopathy or can be secondary to leukostasis, which can occur especially when the white blood cell count is greater than 100 × 10 9 /L (100,000/μL) in patients with leukemia. On MRI, SWI demonstrating low signal and blooming indicates the presence of hemorrhage. Again, leukemia demonstrates homogeneous enhancement, whereas hematomas demonstrate absent or peripheral enhancement.
In a study of 30 intracranial lesions in patients with adult T-cell leukemia, Kitajima and colleagues demonstrated that the most common intracranial lesions were parenchymal lesions, contrast-enhancing (8 lesions in 4 patients), or nonenhancing (8 lesions in 2 patients), followed by leptomeningeal enhancement (5 cases).
Meningeal leukemic infiltration
Leukemia can involve the meninges (pachymeninges, leptomeniges, or both, Figs. 3 and 4 ). One of the commonest early manifestations of CNS leukemia is the symptom of increasing intracranial pressure. Additional symptoms could include cranial nerve palsies, vertigo, ataxia, and myelopathy. The differential diagnosis for leptomeningeal enhancement includes leukemic involvement; however, infectious meningitis or chemical meningitis resulting from intrathecal chemotherapy can also have a similar presentation. In addition, extra-axial granulocytic sarcoma can be mistaken for meningiomas, lymphoma, or extra-axial blood.
Leukemic involvement of the orbit can also present as focal intraconal or extraconal masses, which can involve different components of the globe, lacrimal glands, optic nerve, and extra-ocular muscles. Granulocytic sarcoma can also involve the skull base ( Fig. 5 ) and can present as a paraspinal mass ( Figs. 6 and 7 ) with epidural extension and secondary cord compression, although the prevalence is much less when compared with intracranial involvement. Granulocytic sarcoma of the cord is extremely rare; however, it is reported. Meningeal leukemic involvement can also present in the spine as nerve root enhancement and pial enhancement surrounding the cord (see Fig. 3 ; Fig. 8 ).
Central nervous system disease related to secondary effects of malignancy
Cerebrovascular Complications
A variety of cerebrovascular complications can occur in patients with leukemia, resulting from derangement in hematologic profile, including leukocytosis, thrombocytopenia, coagulopathy, and sepsis. Intraparenchymal hemorrhage is most common in acute leukemia and can be lethal. Patients with acute promyelocytic leukemia are particularly susceptible to disseminated intravascular coagulation during treatment and are susceptible to massive intraparenchymal brain hemorrhage, which also has a high mortality. Leukemic patients with a blast crisis are also susceptible to leukostasis within the small arterioles, which can relate to development of hemorrhagic infarcts ( Fig. 9 ). Extra-axial hemorrhages (subdural or subarachnoid) can also occur in patients with leukemia; however, they are less common.