(1)
Institute of Oncology “Prof. Ion Chiricuță”, Nuclear Medicine & Endocrine Tumors, Cluj-Napoca, Romania
“As the area of light expands, so does the perimeter of darkness”
A. Einstein
10.1 Overview
Neuroendocrine malignant tumors (NET), defined as epithelial neoplasms with predominant neuroendocrine differentiation, arise in most organs of the body. Some of the clinical and pathologic features of these tumors are characteristic of the organ of origin, but other attributes are shared by neuroendocrine neoplasms irrespective of their anatomic site. The multidisciplinary consensus group of experts (North American Neuroendocrine Tumor Society (NANETS), the College of American Pathologists (CAP), the European Neuroendocrine Tumor Society (ENETS)) in the field of NET has recommended a minimum pathology data set of features to be included in pathology reports. The system of classification and nomenclature is depending on the site of the tumor and also of the differentiation grade.
10.1.1 Systems of Nomenclature and Grading System for Neuroendocrine Tumors
10.1.1.1 Lung and Thymus NET (WHO)
Grade of differentiation:
Low grade:
Carcinoid tumors
<2 mitoses/10 hpf (high-power field) and no necrosis
Intermediate grade:
Atypical carcinoid tumors
2–10 mitoses/10 hpf or foci of necrosis
High grade:
Small cell carcinoma
Large cell neuroendocrine carcinoma
>10 mitoses/10 hpf
10.1.1.2 Gastroenteropancreatic (GEP) NET (WHO 2010, ESMO 2012)
Grade of differentiation:
WHO 1-Low grade:
Neuroendocrine neoplasm, grade 1
<2 mitoses/10 hpf and <3% Ki 67 index
WHO 2-Intermediate grade:
Neuroendocrine neoplasm, grade 2
2–20 mitoses/10 hpf and 3–20% Ki 67 index
WHO 3-High grade:
Neuroendocrine carcinoma, grade 3, small cell carcinoma
Neuroendocrine carcinoma, grade 3, large cell neuroendocrine carcinoma
>20 mitoses/10 hpf and >20% Ki 67 index
Mixed adenocarcinoma and neuroendocrine tumor
Tumorlike lesions
Functionally active tumors:
Functionally active NET present with clinical symptoms because of excessive hormone release from the tumor cell
Examples of such events are:
Gastrinoma, excessive gastrin production, Zollinger–Ellison syndrome
Insulinoma, excessive insulin production, hypoglycemia syndrome
Glucagonoma, excessive glucagons production, glucagonoma syndrome
VIPoma, excessive production of vasoactive intestinal peptide (VIP), watery diarrhea-hypokalemia-achlorhydria syndrome
PPoma, excessive PP production (generally classified as nonfunctioning PETs)
Somatostatinoma, excessive somatostatin production
CRHoma, excessive corticotropin-releasing hormones production
Calcitoninoma, excessive calcitonin production
GHRHoma, excessive growth hormone-releasing hormone production
Neurotensinoma, excessive neurotensin production
ACTHoma, excessive production of adrenocorticotropic hormone
GRFoma, excessive production of growth hormone-releasing factor
Parathyroid hormone-related peptide tumor
Functionally inactive tumors:
Functionally inactive NET are diagnosed in several ways:
During routine US performed for the investigation of unexplained upper abdominal complaints
In the case of large tumors of the pancreatic head because of the consequent extrahepatic jaundice
In the case of patients with relapsing abdominal cramps, as a result of intestinal pseudo-obstruction by a functionally inactive NET of the lower jejunum and ileum
As a result of tumor mass symptoms
Genetics:
Non-sporadic NET arise as a result of syndromes caused by an autosomal dominant mutation
Symptoms:
Insulinoma: hypoglycemia
Gastrinoma: peptic ulcer disease
VIPoma: diarrhea
Glucagonoma: necrotic skin rush
Somatostatinoma: mass effect
Carcinoid: flush, rush, diarrhea, sweat
Nonfunctional: mass effects
10.2 Nuclear Imaging of Neuroendocrine Tumors (NET)
Imaging studies for NET are generally performed for an initial evaluation of the extent of the disease and subsequent follow-up. The goals for the initial evaluation include the identification of the primary tumor, staging, and treatment planning. Subsequent follow-up imaging studies are performed for the surveillance after complete resection or during periods of stability and evaluation of response after the treatment.
Imaging modality commonly used includes the following:
Small bowel series
Computed tomography (CT)
Magnetic resonance imaging (MRI)
(In-111 DTPA) octreotide scintigraphy (OctreoScan)
Metaiodobenzylguanidine (MIBG) scintigraphy
Positron emission tomography (PET) and PET/CT
Imaging studies generally recommended at the time of the initial evaluation include plane film radiography of the chest, cross-sectional imaging (CT or MRI) of the abdomen and pelvis, and OctreoScan scintigraphy.
Among patients undergoing surveillance after complete resection, a chest radiography and periodic (every 6–12 months) cross-sectional imaging of the abdomen and pelvis are recommended. For patients having an advanced disease, it is important to evaluate if peptide receptor radionuclide therapy (PRRT) represents a good treatment option.
In-111- and Tc-99m Tektrotyd-labeled somatostatin analogues were developed for the scintigraphy of NET. It shares the receptor-binding profile of octreotide, which makes it a good radiopharmaceutical for imaging of somatostatin receptors 2 and 5.
Imaging is generally performed at 4–6 h and at 24 h. Imaging at 24 or even 48 h provides better contrast because of lower background activity. However, there is often physiological bowel activity that may produce false-positive results. SPECT/CT may be helpful in resolving the nature of indeterminate lesions found on CT and enhances the sensitivity and specificity of the study.
The scintigraphy can be performed for patients on long-acting octreotide but is best performed at the end of the dosing interval (3–6 weeks after the last dose).
For patients on octreotide delivered via a continuous infusion pump or those who received intermittent short-acting octreotide for rescue, it is recommended that these be stopped 48 h before and during testing if possible.
The In-111 octreotide scintigraphy is performed to evaluate the feasibility of PRRT as a scan with intense uptake at all known sites of disease, and it is associated with a higher response rate after radiotherapy with somatostatin receptor targeting.
I-123 MIBG molecular imaging has also been used for NET but has the greatest efficacy in patients with pheochromocytoma, paraganglioma, or neuroblastoma. Some tumors, negative on In-111 octreotide scintigraphy, can be better seen with I-123 MIBG.
Positron emission tomography F18-fluorodeoxyglucose (FDG) imaging, although successful for many solid tumors, has generally not provided additional information about the extent of the disease for well-differentiated NET because of their generally lower proliferative activity.
F18-FDG PET imaging may be used to characterize tumor aggressiveness with higher FDG uptake (expressed as SUV values) having a worse prognosis. This may be helpful when the tumor seems more aggressive than the histology indicates, and additional information for FDG imaging may result in changes of treatment.
Prior studies have shown C11-5-hydroxytryptophan (HTP) PET to be a promising imaging modality for the detection of NET. The serotonin precursor 5-HTP labeled with C-11 was used and showed an increased uptake and irreversible trapping of this tracer in NET. Other new PET imaging agents for NET include F18-DOPA, Ga-68 DOTATOC, Ga-68 DOTANOC, and F18-PGluc-TOCA. In addition, Tc-99m depreotide, which has a greater affinity to somatostatin receptor 3, has also been used for tumor imaging. Although these novel imaging techniques are promising, clinical experiences are limited.
As the latest ESMO guidelines (2012) recommend follow-up, investigations should include biochemical parameters and conventional imaging. In patients with R0/R1 resected NET G1/G2, it is recommended that imaging is performed every 3–6 months (CT or MRI) and, in NEC G3, every 2–3 months. Somatostatin receptor imaging, either OctreoScan or PET/CT using Ga-68 DOTATOC/NOC/TATE, should be included in the follow-up and is recommended after 18–24 months if expression of somatostatin receptor 2A has been proven on the tumor cells. In the case of rapid tumor progression or if imaging information is lacking, it may be necessary to re-biopsy liver metastases to reassess the proliferative activity.
10.2.1 NET Imaging with In-111 OctreoScan
Radiopharmaceutical:
In-111 pentetreotide (OctreoScan)
Principle:
OctreoScan is a radiolabeled analogue of somatostatin indicated for the scintigraphic localization of neuroendocrine tumors bearing somatostatin receptors.
Technique:
Patient preparation:
Bowel preparation, especially for abdominal suspected lesions
Hydration and frequently voiding of the urinary bladder
Attention to diabetic patients where may cause paradoxical hypoglycemia
Temporarily withdrawal of octreotide therapy
Attention to breastfeeding and childbearing age patients (see Chap. 4)
Dose: 3–6 mCi (11–220 MBq)/patient
Injected I.V.
It is necessary to wait 4–6 h after the injection for the first images. Delay images may be registered at 24 h or 48–72 h.
Patient position: supine
Gamma camera
Medium-energy general purpose (MEGP) collimator
Acquisition:
The gamma camera is positioning anteriorly and next posteriorly or simultaneous in dual head camera.
Photon peaks at 172 and 245 keV and 20% windows.
256 × 256 matrix or in whole body 256 × 1024.
Min 500,000 counts/image.
WBS speed 3 cm/min.
Also, spot images on the region of interest and lateral views of head/neck, chest, and abdomen may be obtained..
SPECT is usually recorded with 60 projections of 6° each or 90 projections of 4° each. Matrix 64 × 64..
45–60 s/projection.
Processing: There are special PC programs for image processing; additional analysis of counts in different region of interest (ROI) may be used.
Clinical applications:
Diagnosis and management of receptor bearing gastroenteropancreatic (GEP) neuroendocrine tumors and carcinoid tumors; other non-GEP neuroendocrine tumors
Diagnosis of carcinoid, islet cell carcinoma, gastrinoma, glucagonoma, insulinoma, VIPoma, motilinoma
Necessary additional examinations:
CT, MRI
Ultrasound
Serologic tests of specific tumor markers
Fine needle aspiration biopsy (FNAB)
PET/CT with specific tracers
Comments:
If there is no uptake of OctreoScan, the use of somatostatin analogues in the treatment is discouraged.
SPECT/CT will add superior detection.
Reports:
The report will include a description of the normal distribution and pathologic uptakes; the report should mention the correlation of nuclear findings with the morphological images.
10.2.2 NET Imaging with Tc-99m Tektrotyd
Radiopharmaceutical:
Tc-99m Tektrotyd is a radiopharmaceutical indicated for diagnostics of pathological lesions in which somatostatin receptors are overexpressed (particularly subtype 2 and, to a lesser extent, subtypes 3 and 5) and which may be imaged by the labeled ligand.
Principle:
OctreoScan is a radiolabeled analogue of somatostatin indicated for the scintigraphic localization of neuroendocrine tumors bearing somatostatin receptors.
Technique:
Patient preparation:
Unless there are indications for other methods of the patient preparation, the patient is recommended to stay on light diet 1 day before examination. On the day of examination, the patient should fast until the end of the acquisition. If there is a need for examination after 24 h, it is recommended that a mild laxative be given to the patient starting the evening before. Method of patient preparation may depend on the applied examination protocol and the localization of imaged lesions. However, optimal imaging of abdominal cavity is obtained after the application of liquid diet 2 days before the examination and after administration of laxatives on the day before the examination.
Attention to breastfeeding and childbearing age patients (see Chap. 4).
Dose: 370–925 MBq
Tc-99m Tektrotyd is administered intravenously in a single dose after labeling of the kit using a sterile, oxidant-free sodium pertechnetate solution for injection. Technetium-99m in 1 ml of eluate of sodium pertechnetate Tc-99m solution for injection with activity of 740–1200 MBq (maximally 2200 MBq) may be used for labeling of one kit. This activity is sufficient for examinations of 1–2 adults. Radioactivity of administered dose should be always adjusted with respect to its diagnostic usefulness. The solution may be additionally diluted for more convenient administration.
Patient position: supine
Gamma camera
Low-energy high-resolution (LEHR) collimator
Acquisition:
Acquisition should be carried out between 2 and 4 h after intravenous administration of the preparation. The examination may be complemented by acquisition after 10 min, 1 h, and 24 h after administration of the preparation. It is recommended to carry out the examinations using whole-body technique and SPECT of selected body areas. The gamma camera is positioning anteriorly and next posteriorly or simultaneous in dual head camera.
Photon peaks at 172 and 245 keV and 20% windows; WBS speed 3 cm/min.
Also, spot images on the region of interest and lateral views of head/neck, chest, and abdomen may be obtained.
SPECT is usually recorded with 60 projections of 6° each or 90 projections of 4° each. Matrix 64 × 64.
SPECT/CT should be applied whenever possible for better resolution and sensitivity.
Processing: There are special PC programs for image processing; additional analysis of counts in different region of interest (ROI) may be used.
Clinical applications:
Gastroenteropancreatic neuroendocrine tumors (GEP-NET).
Pituitary adenomas.
Tumors originating in a sympathetic system.
Pheochromocytoma, paraganglioma, neuroblastoma, ganglioneuroma, etc.
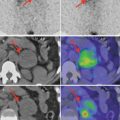
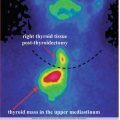
Medullary thyroid carcinoma.
The preparation may be potentially useful in the case of other tumors expressing somatostatin receptors of various intensities.
Other tumors which may overexpress somatostatin receptors: breast cancer, melanoma, lymphomas, prostate cancer, NSCLC, sarcoma, renal cell carcinoma, differentiated thyroid carcinoma, astrocytoma according to WHO I–IV (including glioblastoma multiforme GBM), meningioma, and ovarian cancer.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree