Neuroendocrine Tumors and Nonneoplastic Neuroendocrine Cell Changes
Guido Rindi
Enrico Solcia
INTRODUCTION
Over the last few decades gastric neuroendocrine neoplasms have risen in incidence, in both surgical and endoscopic series. This has been paralleled by a better understanding of the origin and biology of these tumors. In this chapter, we review the normal endocrine cells population of the stomach, their nonneoplastic pathology (hyperplasia and dysplasia), and neoplastic growths, which have traditionally been called “carcinoid” when well-differentiated and “small cell carcinoma” when poorly differentiated. Herein we use the term “neuroendocrine tumor” (NET), which corresponds to neoplasms composed of cells expressing neural antigens, and is now widely used by clinicians for its noncommittal significance regarding malignancy. For consistency, “NET” is used as a synonym of “carcinoid” throughout this chapter. Along the same lines, the term “neuroendocrine carcinoma” (NEC) is used as a synonym for small or large cell neuroendocrine carcinoma to indicate malignant and aggressive poorly differentiated neoplasms.
GASTRIC ENDOCRINE CELLULAR CONTINGENT
Five endocrine cell types are detected in the human gastric mucosa. In the oxyntic mucosa, the most common are the histamine-producing, enterochromaffin-like (ECL) cells, and in the antrum, the gastrin-producing G cells.1,2 Other relatively minor cell populations scattered in both the acidopeptic and the antral mucosa include the serotonin-producing enterochromaffin (EC) cells, the somatostatin D cells, and the ghrelin-producing cells, corresponding to the previously described P/D1 cells.3
PATHOGENESIS AND PREDISPOSING CONDITIONS
Pathogenesis
Long-standing hypergastrinemia is recognized as one of the most important factors leading to endocrine cell hyperplasia and neoplasia in the stomach. Hypergastrinemia is most commonly due to (a) achlorhydria in nonantral (often autoimmune) chronic atrophic gastritis (A-CAG); (b) functioning gastrin cell tumors (gastrinoma) in Zollinger-Ellison syndrome (ZES), with or
without the multiple endocrine neoplasia syndrome type 1 (MEN1); and (c) long-term proton pump inhibitor (PPI) treatment.4, 5 and 6
without the multiple endocrine neoplasia syndrome type 1 (MEN1); and (c) long-term proton pump inhibitor (PPI) treatment.4, 5 and 6
Several lines of evidence support the central role of hypergastrinemia in sustaining ECL cell proliferation. Hypergastrinemia induces increased ECL cell volume density and granule ultrastructure change in CAG patients.6 Alternatively, the cessation of hypergastrinemia following antrectomy induces a dramatic shrinkage of proliferating ECL cells, sometimes with complete disappearance of hyperplastic changes.7
However, hypergastrinemia appears insufficient to promote ECL cell transformation: ECL cell NETs develop in 13% to 43% of MEN1 gastrinoma patients, while developing in <1% of sporadic ZES patients.8 In addition, the absence of proliferative activity in hyperplastic changes, together with follow-up studies in CAG patients, indicates a lack of progression to neoplasia for hyperplastic changes.9 Thus, other factors are probably involved, such as active mediators (cytokines) and growth factors participating in A-CAG inflammatory changes of the gastric mucosa.
Predisposing Conditions
A-CAG and ZES are the conditions most frequently associated with gastric endocrine cell growths. Yet, except in a background of MEN1, ZES patients rarely fail to develop ECL cell dysplastic changes and NETs.10 Pernicious anemia and A-CAG are well-known risk factors for both gastric adenocarcinoma and NETs.11 The frequency of gastric NETs in pernicious anemia/A-CAG patients may range between 1.6% and 10%, with a comparable rate of ECL dysplasia.12
Finally, for sporadic NECs and NETs, that is, developing in nonhypergastrinemic conditions, no specific pathogenetic condition or genetic background has been demonstrated.
NONNEOPLASTIC CHANGES
Observation of gastric endocrine cell changes that do not fit the definition of neoplasia has become more common with increased endoscopic monitoring of dyspepsia, gastritis, and Helicobacter pylori infection, together with the widespread use of PPIs with consequent mild hypergastrinemia. Changes can be observed in both the corpus-fundus and the antrum, often complementing the variable inflammation and atrophy observed in the exocrine mucosa. Mostly, though not exclusively, ECL and G cells changes fit into the categories of hyperplasia and dysplasia.
OXYNTIC MUCOSA
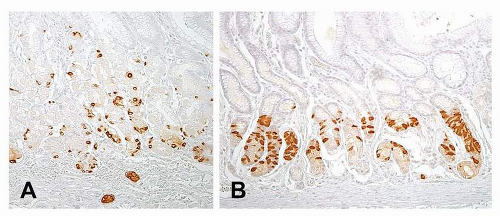
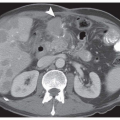
ECL cell hyperplasia and dysplasia have been defined in reference to gastrin-dependent ECL neoplasia as part of a continuum of changes or as precursor lesions (Fig. 7-1A and Table 7-1).13
ECL cell hyperplastic changes (Fig. 7-1A), which lack significant neoplastic potential, are observed within the basal membrane of the gland or in the lamina propria. They are defined as (a) simple (diffuse) hyperplasia, with an increased number of endocrine cells (more than twice Standard Deviation (SD) above normal values) retaining their scattered distribution; (b) linear or chain-forming hyperplasia, characterized by at least five cells in a line along the basal membrane, with a minimum of two chains per linear millimeter of mucosa; (c) micronodular, clusters of at least five cells (30-150 mm in size), either within the basal membrane of the glands or in the lamina propria, with a minimum of one micronodule per linear millimeter of mucosa; and (d) adenomatoid, located within the lamina propria and composed of a minimum of five adjacent micronodules with intervening basal membrane.13
ECL cell dysplastic changes are preneoplastic (precarcinoid) lesions, larger in size (150-500 mm), located in the lamina propria, and defined as (a) enlarging micronodules, which are cellular clusters >150 mm in size; (b) fusing micronodules, apparently resulting from the
disappearance of the basal membranes between adjacent micronodules; (c) microinvasive lesions, infiltrating the lamina propria and filling the space between glands; or (d) nodules with newly formed stroma, displaying a microlobular or trabecular structure.13 ECL cell dysplasia is commonly observed in the mucosa adjacent to ECL cell neoplasia.
disappearance of the basal membranes between adjacent micronodules; (c) microinvasive lesions, infiltrating the lamina propria and filling the space between glands; or (d) nodules with newly formed stroma, displaying a microlobular or trabecular structure.13 ECL cell dysplasia is commonly observed in the mucosa adjacent to ECL cell neoplasia.
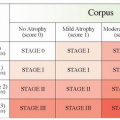
Table 7-1 Endocrine cell hyperplastic, dysplastic, and neoplastic lesion of nonantral stomach | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||
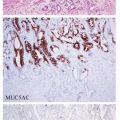
ANTRAL MUCOSA
No dysplastic changes of antral endocrine cells have been described to date; and only hyperplastic alterations are observed.
Antral gastrin cell hyperplasia (Fig. 7-1B) occurs in achlorhydric conditions and has no neoplastic potential. G cell hyperplasia is observed in patients with chronic atrophic gastritis, hypergastrinemia, and hypochlorhydria.14 The lesion is characterized by G cells palisading toward the upper and lower parts of the antral gland. Increased G cells (140-250 per linear mm of mucosa vs. 40-90 in controls) often are associated with reduced D cells, resulting in an abnormal antral G/D cell ratio.15 G cell hyperplasia/hyperfunction and increased G/D cell ratio have been described in children with or without H. pylori infection,16 similar to the changes seen in adults with H. pylori gastritis.17
D cell hyperplasia has been observed in the stomach and duodenum of a patient with dwarfism, obesity, dryness of the mouth, and goiter.18
NEUROENDOCRINE NEOPLASIA
Incidence
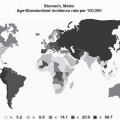
Gastric neuroendocrine neoplasms have risen in incidence in recent series, both surgical and endoscopic. In three large US cancer databases covering from 1950 to 1999, mainly from surgical series, gastric NETs increased from 0.5% to 1.77% of all gastric malignancies (sharply decreasing in absolute figures) and from 2.4% to 8.7% of all gastrointestinal NETs.20 Similarly, a survey of cancer registries of Florida from 1981 to 2000 showed an increase in incidence.21 This increased incidence of gastric NETs fits the incremental trend observed for all types of gastrointestinal NETs in recent years.22,23 Nonetheless, as with nonneoplastic ECL cell changes, the increased figures of gastric NETs may be explained by increased endoscopy and clinical awareness of such lesions. A potentiating role for PPIs still remains to be demonstrated.
Neoplasm Type
NETs represent the largest portion of gastric neuroendocrine neoplasms, while only a minority are NECs. Neoplastic cells with ECL cell characteristics, and, more rarely, G, EC, or ghrelin cells, compose most NETs; other gastric endocrine cell types may also be observed as minor populations.24,25 In a series of 205 gastric neuroendocrine neoplasms, 193 (94%) were NETs, while 12 only were NECs; and 191 out of 193 NETs (98%) were mainly composed of ECL cells, with only 2 representing G cell tumors.26 Rare EC cell tumors27 and, more recently, one “ghrelinoma” have also been described.28 ECL cell NETs are usually located in the corpus-fundus or in the adjacent mucosa extending into the antrum.29
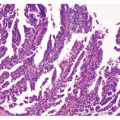
Histology and Grading
Using a series of 102 gastric NETs, a three-tier grading system based on histology and proliferation, has been recently proposed.30 Most cases in this series (81/102), as well as in general practice, display a monomorphic structure with solid nests and tubules, mild cellular atypia, and very few (0-2/10 high power field [HPF]) typical mitoses (Fig. 7-2). Prevalent solid aggregates, scant punctate necrosis, relatively elevated mitotic count (≥7/10 HPF), and moderate cell atypia were observed in rare (5/102) gastric NETs (Fig. 7-2B




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree