Neoplasia
Matthew J. Allen
Neoplasia, literally new growth, is the result of disturbances in cell growth and/or survival. The clinical manifestation of this uncontrolled cell growth is the formation of a tumor. In some cases, for example in the lymph nodes or skin, the tumor may be palpable as a swelling or lump; in many cases, however, the tumor develops in an occult fashion and may be identified only after the patient presents with clinical symptoms. Definitive diagnosis of the lesion as a neoplasm depends on cytological or histological examination of a representative biopsy sample. At the same time, the pathologist will also determine whether the tumor is benign (unlikely to spread, or metastasize) or malignant (likely to metastasize). The term cancer, although generally used to describe all tumors, should be restricted to neoplastic lesions with malignant potential.
Prevalence
Epidemiologic data for cancerous neoplasms, while only the tip of the iceberg in terms of overall neoplasms, are much more readily available.
Cancer is the second most common cause of death in the United States (heart disease remains the number-one cause of mortality).
According to the American Cancer Society, over 291,000 men and 273,000 women are expected to die as a result of cancer in 2006.
Breast and prostate cancers are the most common cancers, but lung cancer remains the most lethal.
Overall, the lifetime risk of developing cancer is 1 in 2 for men and women in the United States.
Etiology
It is generally accepted that some degree of chromosomal abnormality is required for cancer to develop. Most cancer cells display evidence of defects in either chromosome number or composition, and it is presumed that these genomic changes lie at the heart of the cancer phenotype. However, with relatively few exceptions, the mechanisms through which these alterations lead to cancer remain unclear. Cancer is a disease that develops by clonal expansion from a single abnormal cell. The underlying abnormality is typically genetic, but with successive cycles of cell division there is potential for additive damage through both genetic and nongenetic mechanisms. The end result of these changes is an expanding clone of cells that is unresponsive to normal growth controls.
Loss of Normal Growth Control in Cancer Cells
Normal cellular growth is controlled by four major groups of regulatory proteins. Changes in the level of expression or in the genetic sequence of these regulatory proteins have the capacity to alter their function and, as a result, disturb normal cellular growth.
Major groups of regulatory proteins are involved in normal cellular growth control.
Growth factors
Definition: circulating factors or local factors that act on cells with specific receptors
Mechanisms of disturbance: Inappropriate expression (up- or down-regulation) can lead to uncontrolled cell growth.
Example: Disturbances in fibroblast growth factor (FGF) have been implicated in the development of sarcomas.
Growth factor receptors
Definition: Typically expressed either on the cell surface or within the cytoplasm, these receptors bind and transduce external signals from growth factors into intracellular signals.
Mechanisms of disturbance: Disturbances in receptor activity may increase or decrease receptor numbers on the cell, or they may involve dysregulated receptors that are permanently “on,” even in the absence of the growth factor signal.
Example: Overexpression of c-erb2/HER2 is implicated in breast cancer.
Intracellular signaling proteins
Definition: These proteins transfer the signal from surface receptors to the nucleus, where the target is most often a nuclear transcription factor (see below).
Mechanisms of disturbance: Overactivity of these signaling proteins, for example as a result of constitutive overexpression of the gene, leads to uncontrolled nuclear signaling and activation of downstream genes, many of which regulate cellular growth and/or survival.
Example: Overexpression of the ras family of oncogenes is found in many tumors.
Nuclear transcription factors
Definition: These control elements act directly on DNA, resulting in gene transcription.
Mechanisms of disturbance: In some cases chromosomal damage directly affects the expression of the transcription factor, while in others the effects are mediated via changes in the expression of tumor suppressor proteins.
Examples: A number of transcription factors have been implicated in human cancer, including E2F (involved in the cell cycle), p53, and MDM2 (see below).
Downstream effects of mutations in any of these control elements
Depend on the nature of the affected gene and the extent of the change
Defects in one copy of a tumor suppressor gene may have no clinical consequence as long as the second copy is normal.
Defects in the androgen receptor may be silent in females but cause feminization in males.
In the most extreme cases, defects in just one copy of a gene may still lead to clinical disease.
Example: autosomal-dominant conditions such as achondroplasia (associated with a defective gene for the FGF3 receptor)
Genetic Alterations in Cancer
Mutations and Cancer
Definition of mutation: changes in the normal sequence of DNA as a result of defects in DNA replication
Causes of mutations
Endogenous defects in DNA replication and/or DNA repair
Exogenous factors such as radiation or chemical carcinogens
Timing of mutations relative to cell division
Only at the time of DNA replication is damage converted into a change in the DNA sequence.
Nondividing cells are less susceptible to mutation than are cells that are highly proliferative (e.g., hematopoietic tissues, skin, intestinal tract).
Targets for genetic mutation in cancer
Typically regulatory elements that affect cellular growth, differentiation, and survival (see above)
Clinical importance of mutations
Associations between a particular cancer and a specific pattern of mutation exist.
Associations are rarely absolute.
Used in some cases to confirm histological diagnoses
Example: 11:22 translocation in Ewing sarcoma
It is common to find that an individual tumor contains multiple gene defects, and it is the combination of defects, rather than any single mutation, that determines the phenotype of the tumor in terms of growth, risk of metastasis, and response to therapy.
Examples: conventional osteosarcoma, chondrosarcoma
Cancer and the Cell Cycle
As described in Chapter 13, Cellular and Molecular Biology, the cell cycle is regulated by a complex series of control elements that ensure the integrity of the DNA, the accuracy of DNA replication, and the integrity of the cytoskeletal elements that facilitate the division of the two sets of DNA into the daughter cells. Disturbances in one or more of these control elements can lead to a spectrum of problems, including decreased proliferative capacity, abnormal cellular differentiation, resistance to apoptosis, or unchecked cellular proliferation. It is therefore not surprising that disturbances in the normal cell cycle lie at the heart of many human cancers.
Cyclins and Cyclin-Dependent Kinases (Cdks) (Fig. 28-1)
Critical role in controlling the orderly progression of cells through the cell cycle
Mammalian species have at least 10 cyclins (cyclins A, B, C, etc.).
Cyclins cannot act alone.
Require activation by binding of a cyclin-dependent kinase (Cdk) to form Cdk—cyclin complex
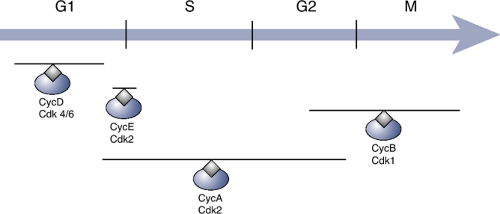
Figure 28-1 Regulation of the cell cycle by cyclins and cyclin-dependent kinases. The gray lines above each complex indicate the stages of the cell cycle during which they are most active.
In mammalian species there are at least six Cdks (Cdk 1 through 6).
Subsequent phosphorylation of the Cdk within the Cdk—cyclin complex
Phosphorylating enzyme known as Cdk-activating kinase (CAK)
Sequence in mammalian cells
Cell cycle initiated by binding of cyclin D to either Cdk4 or Cdk6
Final phase catalyzed by binding between cyclin B and Cdk1 to produce a complex known as mitosis-promoting factor (MPF)
Control over cyclin—Cdk activity exerted through effects at multiple levels
Cyclin synthesis or breakdown
Cyclin—Cdk binding
CAK activity
Direct inhibition of Cdk activity by Cdk inhibitors
Proto-Oncogenes, Cellular Oncogenes, and Viral Oncogenes
Oncogenes
DNA sequences that regulate the growth of tumor cells
Derived from proto-oncogenes
Proto-oncogenes
DNA sequences in the healthy cell that encode transcription factors, intracellular signaling pathways, and receptors that control normal cellular growth, differentiation, and apoptosis
Importance of these genes as regulatory elements
Highlighted by the fact that they have been highly conserved throughout the course of evolution
Also strong homology between proto-oncogenes in different vertebrate species and even nonvertebrates (e.g., Drosophila, yeast species)
Conversion of a proto-oncogene into an oncogene: This key step in cancer initiation involves activation through one of three mechanisms:
Point mutation: Changes in the nucleic acid sequence can render the gene product resistant to inhibitory elements.
Translocation: If the oncogene becomes associated with a new promoter, it may be expressed at abnormally high levels. Alternatively, combination of the oncogene with a gene sequence can lead to the formation of a fusion gene whose product can enhance cellular growth.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree