Neck Cancer Including Unknown Primary Tumor
 ANATOMY
ANATOMY
The locations of the various lymph node groups in the head and neck are shown in Figure 49.1.1 Under normal conditions, the right and left lymphatic networks do not shunt from one side to the other.2
The internal jugular chain (IJC) lymph nodes lie adjacent to the internal jugular vein and extend from the skull base to the clavicle. The most superior group of lymph nodes in this chain lies near the base of the skull in the posterior aspect of the lateral pharyngeal space and is often referred to as the parapharyngeal or junctional lymph nodes. These lymph nodes lie deep to the sternocleidomastoid muscle, the posterior belly of the digastric muscle, and the tail of the parotid gland. The remaining IJC lymph nodes are artificially divided into the subdigastric, middle jugular, and lower jugular groups.
The spinal accessory chain (SAC) lymph nodes are distributed along the course of cranial nerve XI. The superior nodes of the SAC blend with the upper IJC nodes. The supraclavicular lymph nodes merge laterally with the SAC lymph nodes and medially with the lower IJC lymph nodes.
There are three to six submandibular lymph nodes. They may be either preglandular or postglandular; there are no lymph nodes in the substance of the submandibular gland. The submental lymph nodes lie in the midline between the anterior bellies of the digastric muscles, anterior to the hyoid bone and external to the mylohyoid muscle. The lateral retropharyngeal lymph nodes lie within the retropharyngeal space, which is bounded anteriorly by the pharyngeal constrictor muscles, superiorly by the skull base, and posteriorly by the prevertebral fascia. They are usually at the level of the C1 and C2 vertebral bodies but may be found as inferiorly as C3. The medial retropharyngeal nodes are small, inconstant intercalated nodes that are located near midline and empty into the lateral retropharyngeal lymph nodes.
The neck nodes are divided into levels as follows: level I, submental (IA) and submandibular (IB) nodes; level II, upper internal jugular nodes, from the skull base to the level of the hyoid bone; level III, middle internal jugular nodes, from the level of the hyoid bone to the omohyoid muscle; level IV, inferior internal jugular nodes, from the level of the omohyoid muscle to the clavicle; level V, spinal accessory lymph nodes; and level VI, anterior neck nodes, bounded by the hyoid bone, the sternum, and the common carotid arteries. Included in level VI are the paratracheal, pretracheal, precricoid (Delphian), and tracheoesophageal groove nodes.3
FIGURE 49.1. Arrangement of lymph nodes in the head and neck. (Redrawn from Rouviere H. Anatomy of the human lymphatic system, Tobias MJ [trans]: Ann Arbor, MI: Edwards Brothers, 1938:27.)
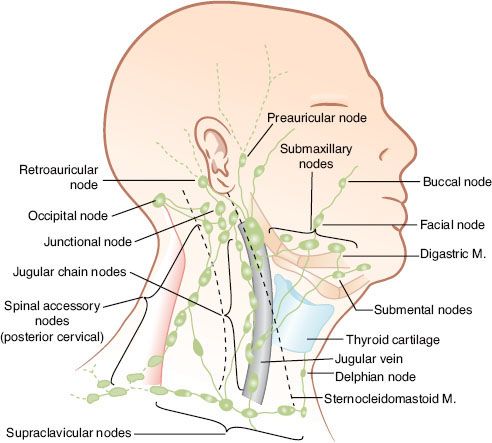
TABLE 49.1 DEFINITION OF RISK GROUPS

 NATURAL HISTORY
NATURAL HISTORY
The risk of lymph node metastases is influenced by the location of the primary tumor, histologic differentiation, size of the lesion, and the availability of capillary lymphatics.4–7 The estimated risk of subclinical disease in the clinically negative neck as a function of primary site and tumor (T) stage is shown in Table 49.1.4 Recurrent lesions have a higher risk of lymphatic involvement than untreated lesions.
The relative incidence of clinically positive lymph nodes in the neck by anatomic site and T stage is shown in Table 49.2.5 The most commonly involved lymph nodes in the head and neck are the level II lymph nodes, followed by the level III lymph nodes. Lesions that are well lateralized almost always spread first to the ipsilateral neck nodes. Lesions on or near the midline as well as lateralized base of tongue and nasopharyngeal lesions may spread to both sides of the neck.
Patients who have clinically positive lymph nodes on the ipsilateral side of the neck may be at risk for contralateral lymph node spread if the metastatic masses produce significant obstruction of the lymphatic trunks. In addition, patients who have undergone previous surgery on one side of the neck develop shunting of lymph across the submental region to the opposite side of the neck. When contralateral lymph node metastases occur, the level II lymph nodes are most frequently involved, followed by the level III and level IV lymph node groups.
As tumor grows within a lymph node, the node becomes indurated and more rounded, and enlarges. Tumor eventually extends through the capsule of the lymph node and invades surrounding structures. Extension to the neurovascular bundle is common and may produce a mass that is considered fixed to palpation. The incidence of tumor involvement and the likelihood of capsular penetration as a function of lymph node size are shown in Table 49.3.6
The risk of lateral retropharyngeal lymph node involvement is related to primary site and neck stage7; the medial retropharyngeal nodes are almost never the site of metastatic disease. The incidence of positive retropharyngeal nodes based on pretreatment computed tomography (CT) and, in selected cases, magnetic resonance imaging (MRI) is shown in Table 49.4.7
 DIAGNOSTIC WORKUP
DIAGNOSTIC WORKUP
Physical Examination
The patient is examined in the sitting position, the examiner behind the patient with one hand on the occiput to flex the patient’s head forward and the other hand on the side of the neck to be examined. To examine the IJC lymph nodes, which lie deep to the sternocleidomastoid muscle along the internal jugular vein, place the thumb and index finger around the sternocleidomastoid muscle in the form of a “C” and then gently proceed from the sternal notch to the angle of the mandible. Both sides of the neck should not be examined simultaneously. The level Ib and level Ia nodes may be evaluated by direct palpation of these areas as well as by a bimanual examination with the index finger placed in the floor of the mouth.8
The following features of metastatic lymph nodes should be recorded: anatomic location, size, consistency, mobility, and clinical impression as to whether the node is involved with cancer.
TABLE 49.2 CLINICALLY DETECTED NODAL METASTASES ON ADMISSION CORRELATED WITH T STAGE
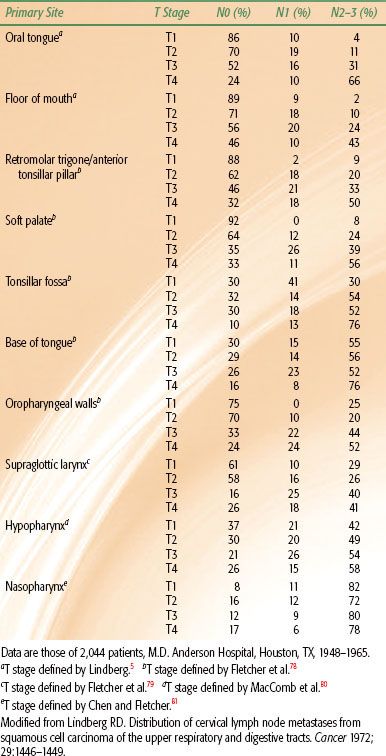
TABLE 49.3 RELATIONSHIP BETWEEN NODE SIZE, THE PRESENCE OF TUMOR IN THE NODE, AND CAPSULAR PENETRATION IN 519 NODESA
Radiographic Evaluation
CT, MRI, fluorodeoxyglucose–positron emission tomography (FDG-PET), and ultrasound may be used to evaluate cervical metastatic disease.9 At the University of Florida, CT remains the primary method of examination of most carcinomas arising in the upper aerodigestive tract and the regional lymphatic system. MRI is the primary study only in patients with nasopharyngeal malignancies. MRI also may be used in patients who are allergic to intravenous contrast medium. Ultrasound has been used mainly in Europe to evaluate the cervical nodes. FDG-PET may be used to evaluate equivocal suspicious lymph nodes if the results of the scan would alter the treatment plan. Positive nodes <1 cm will not be reliably detected on a PET scan. FDG-PET remains unproven with regard to improving accuracy rates over those available with properly performed and interpreted CT.
Small metastases may be seen as lucent foci in normal-sized nodes. Such metastases have been identified and surgically confirmed in nodes as small as 6 to 8 mm; however, most subclinical disease in normal-sized nodes remains undetected on CT. FDG-PET has a marginal capability to improve on CT in detecting the subclinical disease in small (<1 cm) nodes.
Lucent foci in normal-sized nodes must be differentiated from hilar fat or volume-averaging artifacts. As the metastasis grows, the node becomes more spherical than elliptical. Areas of necrosis are almost always present in nodal metastases larger than 2 cm. As the metastasis enlarges, the capsule of the node becomes hyperemic and is seen radiographically as a contrast-enhanced rim. When the capsule becomes indistinct and irregular along its outer margin, it is highly suggestive of early capsular penetration. Continued growth causes obliteration of the fat planes surrounding the nodes. Finally, no clear plane of normal tissue lies between the mass and the adjacent structures, at which point the clinician usually notes fixation (Fig. 49.2). Penetration of the prevertebral fascia and fixation to the scalene muscles are uncommon in untreated patients. Largely necrotic nodes may be negative on FDG-PET examinations.
If a node shows evidence of capsular penetration and envelops more than 50% of the circumference of the carotid artery, clinical evidence of fixation to the artery is likely. Ultrasound and MRI may prove useful in evaluating tumor extension to the carotid, as suggested by CT. MRI tends to be better at excluding extension to the neurovascular bundle when it is suspected on CT, whereas ultrasound can help show invasion of the vessel wall, thus confirming focal extension to the artery.
FIGURE 49.2. T1 squamous cell carcinoma of the lateral wall of the right pyriform sinus (open arrow) and a fixed N3B neck node that abuts but does not surround the carotid artery (solid arrow). Mendenhall WM, Parsons JT, Mancuso AA, et al. Head and neck: management of the neck. In: Perez CA, Brady LW, Halperin EC, et al., eds. Principles and practice of radiation oncology. Philadelphia: Lippincott Williams and Wilkins 2004:1158–1178.

TABLE 49.4 INCIDENCE OF POSITIVE RETROPHARYNGEAL NODES FOR VARIOUS PRIMARY SITES AND CLINICAL NECK STAGES (794 TUMORS)

 STAGING
STAGING
The staging systems shown in Table 49.5 are those of the American Joint Committee on Cancer (AJCC). Because all University of Florida data presented in this chapter were analyzed using the 1983 AJCC staging system,10 both the 1983 and 2010 systems are outlined in Table 49.5. Stage N3C in the 1983 system is rare and should alert the clinician to search for another primary lesion. The 2010 AJCC staging system classifies bilateral or contralateral nodes not more than 6 cm in diameter as N2C; N3 is defined as a metastasis in a lymph node more than 6 cm in diameter.11 The AJCC nodal staging system for head and neck cancer has not changed appreciably since the 1997 edition. The AJCC nodal staging for nasopharyngeal carcinoma differs from other head and neck primary sites and will be discussed in the chapter devoted to that topic.
 SURGERY
SURGERY
Standard radical neck dissection involves removal of the superficial and deep cervical fascia with its lymph nodes in levels I to V in continuity with the sternocleidomastoid muscle, omohyoid muscle, internal and external jugular veins, spinal accessory nerve, and submandibular gland. Sacrifice of cranial nerve XI often, but not always, results in atrophy of the trapezius muscle, with shoulder drop and discomfort.
Modified radical neck dissection removes the superficial and deep cervical fascia with its enclosed lymph nodes and leaves one or more of the nonlymphatic structures such as the sternocleidomastoid and digastric muscles, internal jugular vein, and spinal accessory nerve. Currently, almost all of the patients treated with neck dissection at the authors’ institution undergo this operation with at least preservation of cranial nerve XI. The advantages of the functional neck dissection are less cosmetic deformity and better function.
For a selective neck dissection, one or more of lymph node groups I to V are not removed. The advantage of the selective neck dissection is that it provides equivalent efficacy and less morbidity in appropriately selected cases. Supraomohyoid neck dissection removes the lymph nodes in levels I to III and is most commonly used for patients with small oral cavity cancers and a clinically negative neck. The lateral neck dissection entails removal of level II to IV nodes and is most often used in the treatment of laryngeal, oropharyngeal, and hypopharyngeal cancers. If significant metastatic adenopathy is encountered during a selective neck dissection, it should be converted to a radical or modified radical dissection.
An extended radical neck dissection implies removal of additional lymph node groups or nonlymphatic structures in addition to the structures removed in a radical neck dissection. Bilateral neck dissections may be performed simultaneously or separately (staged) in patients with bilateral neck disease as long as one internal jugular vein can be preserved. At one time, simultaneous neck dissection appeared to be associated with a higher incidence of complications and operative mortality compared with staged neck dissections.12,13 The authors’ more recent experience suggests that this is no longer the case.
Complications of Neck Dissection
Complications of neck dissection include hematoma, seroma, lymphedema, wound infection, wound dehiscence, chyle fistula, damage to cranial nerves VII, X, XI, and XII, carotid exposure, and carotid rupture. The incidence of complications is higher when neck dissection is combined with resection of the primary lesion or when it follows a course of radiation therapy (RT). The postoperative mortality rate for unilateral neck dissection after RT was 3% for patients treated between 1964 and 1982.14
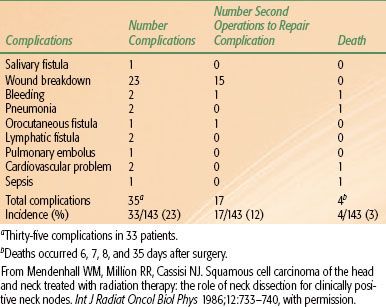
The incidence of postoperative complications in a series of patients treated with RT to the primary lesion and neck followed by unilateral or bilateral neck dissection(s) is shown in Tables 49.614 and 49.7.15 Two of 10 patients undergoing a staged bilateral neck dissection experienced a moderately severe complication compared with 4 of 40 patients undergoing a simultaneous bilateral neck dissection. None of the 10 patients who underwent a staged bilateral neck dissection experienced a severe complication, compared with 6 of 40 patients (15%) who underwent a simultaneous bilateral neck dissection (P = .24).15
Taylor et al.16 analyzed the incidence of moderate (2+) and severe (3+) wound complications in a series of 205 patients who underwent a planned unilateral neck dissection after RT at the University of Florida. RT was given once daily in 123 patients, twice daily in 80 patients, and with both techniques in the remaining two patients. The incidence of wound complications increased with total dose and dose per fraction (Fig. 49.3).
FIGURE 49.3. Complication rate (2+ or 3+) versus total dose. Separate analysis for once a day (filled square, solid curve) and twice a day (circle, dashed curve). Data are plotted at the midpoints of the range 45 to 60 Gy, 60 to 70 Gy, and 75 to 90 Gy. Error bars denote 95% confidence intervals. The curves are the results of separate logistic regression analysis. (From Taylor JMG, Mendenhall WM, Parsons JT, et al. The influence of dose and time on wound complications following post-radiation neck dissection. Int J Radiat Oncol Biol Phys 1992;23:41–46, with permission.)

TABLE 49.5 1983 AND 2010 AMERICAN JOINT COMMITTEE ON CANCER STAGING FOR NECK LYMPH NODES

TABLE 49.6 POSTOPERATIVE COMPLICATIONS OF UNILATERAL NECK DISSECTION AFTER IRRADIATION TO THE PRIMARY LESION AND NECK (143 PATIENTS)
 RADIATION THERAPY
RADIATION THERAPY
RT may be used in the treatment of cervical lymph node metastases as elective treatment when there are no palpable lymph nodes, as the only treatment for clinically positive lymph nodes,17 or as preoperative or postoperative treatment in combination with neck dissection for clinically positive lymph nodes.18
The regional lymph nodes are considered in the treatment planning of the primary lesion. With clinically negative neck nodes, treatment planning depends on the estimated risk of subclinical disease in the nodes. With clinically positive lymph nodes, the plan is influenced by the number of lymph nodes, size, and location.
Elective Radiation Therapy of Cervical Lymph Nodes When the Primary Tumor Is Treated by Radiation Therapy
Factors that influence the decision to irradiate the neck electively are site and size of the primary lesion, histologic grade, difficulty in neck examination, relative morbidity for adding lymph node coverage, likelihood of the patient’s returning for follow-up examinations, and suitability of the patient for a radical neck dissection if the tumor appears in the neck at a later date. Patients in whom the primary lesion is to be treated by RT, who have clinically negative nodes, and in whom the risk of subclinical disease is 20% or greater usually receive elective neck irradiation to a minimum dose equivalent to 45 to 50 Gy over 4.5 to 5 weeks (see Table 49.1). Patients with lesions arising in the lip, nasal vestibule, nasal cavity, or paranasal sinuses have a low risk of subclinical neck disease, and the neck is not treated electively unless the lesion is recurrent, advanced, or poorly differentiated. Similarly, the risk of occult neck disease is essentially 0% for T1 and 1.7% for T2 glottic carcinomas, and elective neck radiation therapy is not indicated.19,20
The lateral treatment portals used to encompass cancers in the oropharynx, supraglottic larynx, and hypopharynx include the upper jugular and often the midjugular chain lymph nodes. RT portals used for primary lesions of the oral cavity, nasopharynx, glottis, nasal cavity, and paranasal sinuses must be enlarged to include the lymph nodes. The treatment portals for irradiation of the cervical lymph nodes must be designed in such a way as to minimize additional mucosal irradiation. A common error in irradiating oropharyngeal and nasopharyngeal cancers is to enlarge the lateral (primary) portals inferiorly to unnecessarily include all of the larynx in the lateral portals (Fig. 49.4).21 Because the midneck is smaller in circumference than the upper neck, the total dose and dose per fraction are higher in the larynx than along the central axis of the beam, leading to double trouble. Although a field junction through a positive node(s) may be avoided with intensity-modulated radiation therapy (IMRT), the larynx still receives a substantially higher dose compared with a separate anterior low neck portal with a midline laryngeal block junctioned at the thyroid notch (Figs. 49.5, 49.6, and 49.7).22 Treating an unnecessarily large field increases the acute and late effects of RT and, by increasing the risk of an unplanned split, reduces the probability of disease control.21,22
IMRT may be used to treat patients if there is a goal that can be achieved to reduce the toxicity of irradiation. These goals are usually parotid sparing to reduce the risk of long-term xerostomia, avoiding a low neck match in patients with a low-lying larynx, and improved coverage of the poststyloid parapharyngeal space in patients with nasopharyngeal cancer.23 If one or more of these goals cannot be achieved, the patients may be better off being treated with conventional RT because of the disadvantages of IMRT, which include increased risk of a marginal miss, less homogeneous dose distribution, and increased cost and complexity.23
Elective neck irradiation for early oral cavity lesions includes the level Ib and level II lymph nodes. The level III and level IV lymph nodes are treated as well by using a narrow anterior field. For primary lesions located in the oropharynx, nasopharynx, supraglottic larynx, and hypopharynx, the lower neck nodes are also routinely included. The low neck is treated with a single anterior field (Fig. 49.8). A tapered midline larynx or trachea shield is added to protect the spinal cord, the larynx, and the pharynx. For primary lesions lying below the thyroid notch, a small midline tracheal block 5- to 10-mm wide is placed in the low-neck field, primarily to avoid field overlap at the spinal cord. A 1-cm wide midline block made of Lipowitz’s metal may be used to shield the trachea, esophagus, and spinal cord below the level of the cricoid.
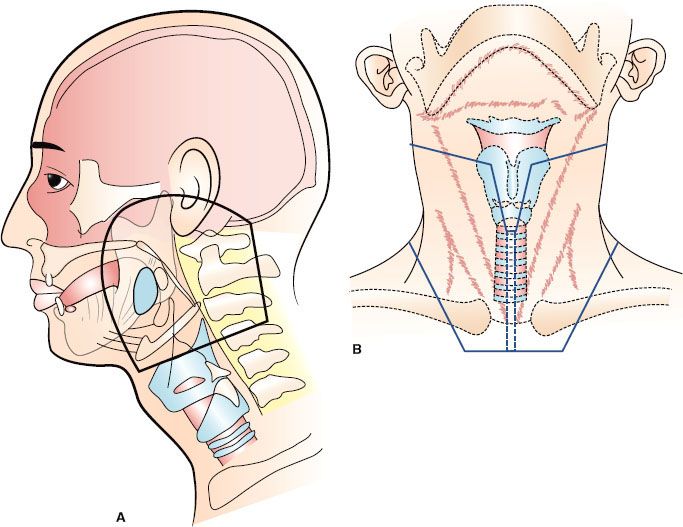
FIGURE 49.4. Carcinoma of the base of the tongue: large radiation portals. A: Parallel-opposed lateral portals include the primary lesion, larynx, hypopharynx, most of the cervical spinal cord, and the upper portion of the trachea and cervical esophagus. Treatment through this portal tangentially irradiates the skin of the anterior neck unnecessarily. If an anterior field is not used to irradiate the low neck, the inferior border of the lateral field may be placed near the clavicle (dashed line). B: Anterior low-neck portal. The wide midline tracheal block partially shields the low internal jugular lymph nodes, which are located adjacent to the trachea. The supraclavicular lymph nodes, which are less likely to be involved with tumor than the low jugular nodes, are adequately covered. C: Central axis dosimetry at the level of the base of tongue primary lesion. The contours were obtained from a RANDO phantom using parallel cobalt-60 fields weighted equally. The base of tongue tumor is outlined, and the tumor dose is specified at 97% of maximum dose. D: Off-axis contour through larynx. The minimum dose to the entire larynx is 104% of the maximum dose specified at the central axis, and the maximum dose on this off-axis contour is 113%. If the base of tongue tumor dose is specified as 50 Gy at 2 Gy per fraction, the minimum larynx dose is 53.61 Gy at 2.14 Gy per fraction, and the maximum larynx dose is 58.25 Gy at 2.33 Gy per fraction. If the tumor dose is specified as 60 Gy at 2 Gy per fraction, the minimum larynx dose is 64.33 Gy at 2.14 Gy per fraction, and the maximum larynx dose is 69.9 Gy at 2.33 Gy per fraction. (From Mendenhall WM, Parsons JT, Million RR. Unnecessary irradiation of the normal larynx [editorial]. Int J Radiat Oncol Biol Phys 1990;18:1531–1533, with permission.)

FIGURE 49.5. Laryngeal dose in a model patient with a stage T2N2b carcinoma of the tonsil with positive nodes on the right side at the level of the larynx. The primary site is irradiated with either intensity-modulated radiation therapy or lateral opposed fields. The cervical lymphatics inferior to the primary site fields are treated with an anterior low-neck field. A: Digitally reconstructed radiograph of the low-neck fields. The larynx was contoured and appears as a red color-wash structure. The larynx is shielded with a narrow midline block that does not cover the entire width of the larynx. In this model patient, the entire low-neck field received 50 Gy, and then the field size was reduced to boost the positive nodes on the right of the larynx to 70 Gy. Irradiation was given with a 6-MV photon beam with source to axis distance of 100 cm. B: Axial dose distribution at the level of the true vocal cords showing that the dose to the central portion of the larynx is extremely low when the larynx is shielded in the anterior low-neck field. (From Amdur RJ, Li JG, Liu C, et al. Unnecessary laryngeal irradiation in the IMRT era. Head Neck 2004;26:257–264, with permission.)
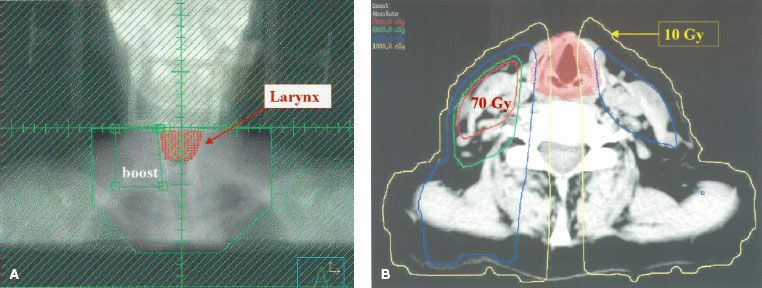
FIGURE 49.6. Dose distribution using intensity-modulated radiation therapy as described in the text to treat the model patient with a stage T2N2b carcinoma of the tonsil with positive nodes on the right side at the level of the larynx. The plan was optimized to minimize the dose to the larynx while delivering 70 Gy to gross disease and 59.4 Gy to areas at risk for subclinical disease. A: Coronal projection near the middle of the larynx. B: Axial projection at the level of the true vocal cords. A comparison of Figures 49.5B and 49.6B shows that sparing of the central portion of the larynx is shielded in an anterior low-neck field. (From Amdur RJ, Li JG, Liu C, et al. Unnecessary laryngeal irradiation in the IMRT era. Head Neck 2004;26:257–264, with permission.)

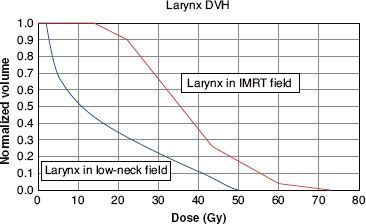
FIGURE 49.7. Dose–volume histogram of the larynx for the model patient described in Figures 49.5 and 49.6. The thicker line is the dose–volume histogram when the larynx is included in the intensity-modulated ration therapy (IMRT) fields shown in Figure 49.6. The thinner line is the dose–volume histogram when the larynx is shielded in the anterior low-neck field shown in Figure 49.5. There is a major difference in the portion of the larynx that receives an extremely low dose. For example, when the larynx is included in the IMRT fields, the entire larynx receives more than 10 Gy, whereas when the larynx is shielded in the low-neck field, approximately 45% of the larynx receives <10 Gy. (From Amdur RJ, Li JG, Liu C, et al. Unnecessary laryngeal irradiation in the IMRT era. Head Neck 2004;26:257–264, with permission.)
Treatment of Clinically Positive Cervical Lymph Nodes When the Primary Tumor Is Treated by Radiation Therapy
The dose required to control a clinically positive lymph node that is included within the RT portals depends on the size of the lymph node17,24 and whether concomitant chemotherapy is administered. Relatively recent data suggest that advanced disease has a better chance of cure after altered fractionation or concomitant chemotherapy.25 Patients treated at the authors’ institution routinely receive hyperfractionation when using three-dimensional conformal RT and the concomitant boost technique when using IMRT, combined with weekly cisplatin 30 mg/m2. Positive nodes receive approximately 70 to 74 Gy, regardless of size or rate of regression.
The decision to add a neck dissection after RT for multiple unilateral positive nodes or bilateral lymph node disease is individualized and is based on the diameter of the largest node, node fixation, and number of clinically positive nodes in the neck. If clinically positive lymph nodes disappear completely during RT, the likelihood of control by RT alone is improved and a neck dissection may be withheld.26–29 Peters et al.30 reported on 100 node-positive patients with squamous cell carcinoma of the oropharynx treated with concomitant boost RT between 1984 and 1993 at the MD Anderson Cancer Center (Houston, TX). Sixty-two patients had a complete response in the neck and received no further therapy. Three patients (5%) subsequently developed an isolated recurrence in the neck and four patients (6%) developed a recurrence in the neck in conjunction with other sites of relapse. The 2-year neck disease control rates did not vary significantly with pretreatment nodal size: ≤3 cm, 87%; and >3 cm, 85%. The incidence of subcutaneous fibrosis was similar following RT alone compared with another group of patients who underwent a neck dissection in addition to RT. Johnson et al.31 reported on 81 patients with node-positive stages III and IV squamous cell carcinoma of the head and neck treated with concomitant boost accelerated hyperfractionated RT at the Medical College of Virginia (Richmond). Fifty-eight patients (72%) had a complete response in the neck and were followed; three patients (5%) subsequently developed an isolated recurrence in the neck and one additional patient developed recurrent cancer in the neck and in the primary site. The 3-year neck disease control rates were 94% for nodes ≤3 cm compared with 86% for those >3 cm.
Both of these series of patients received aggressive altered fractionated RT and it is unclear whether these data can be broadly extrapolated to patients with head and neck cancer from a variety of head and neck primary sites that are treated less aggressively. It is also unclear whether the addition of concomitant chemotherapy results in a lower likelihood of needing a neck dissection. However, multiple subsequent studies evaluating neck control rates after RT alone or combined with chemotherapy suggest that the likelihood of an isolated failure in the neck is low if there is a complete response after treatment.18,32–36
FIGURE 49.8. Lateral and anterior fields are used to irradiate a patient with a carcinoma limited to the base of tongue. A: Parallel-opposed fields include the primary lesion with a 2- to 3-cm inferior margin. The lower border of the field is placed at the thyroid notch and slants superiorly as the junction line proceeds posteriorly as the junction line proceeds posteriorly. This substantially reduces the amount of mucosa larynx and spinal cord included in the primary treatment portals. B: En face low-neck portal with tapered midline larynx and tapered midline larynx block. It is not necessary to treat the supraclavicular fossa unless clinically positive nodes are found in that particular hemineck. A 5-mm midline tracheal block may be placed in the low-neck portal (dashed line). (From Mendenhall WM, Parsons JT, Million RR. Unnecessary irradiation of the normal larynx [editorial]. Int J Radiat Oncol Biol Phys 1990;18:1531–1533, with permission.)