TABLE 7.1 Adverse Morphologic Prognostic and Predictive Factors of Bladder Cancer | ||||||
|---|---|---|---|---|---|---|
|
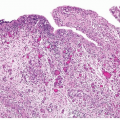
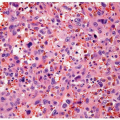
grade, stage, and mortality increases from low-grade carcinomas to highgrade carcinomas. High-grade disease is a stratifier for management and therapeutic decisions in the recent NCCN guidelines for noninvasive tumors (6). Glandular differentiation may occasionally occur in noninvasive tumors, and approximately 50% of these patients develop invasive carcinoma, although the invasive component is unlikely to be adenocarcinoma (2, 7, 8).
correlation with prognosis and distinguishes distinct prognostic groups. Tumors invasive into the lamina propria (pT1) have a better survival than tumors invasive into the muscularis propria (pT2), with poor survival for tumors with extravesicular extension (pT3, pT4) (1, 22, 23, 24). Although substaging of urothelial tumors (pTla-tumors invasive up to muscularis mucosae, pTlb-tumors invasive into or beyond muscularis mucosae) has shown prognostic value, it is currently not recommended because it may not always be possible to substage pT1 tumors due to the absence of muscularis mucosae in some bladders or because of a lack of orientation in transurethral resection specimens precluding orientation (4). Chapter 5 outlines the diagnostic criteria, pitfalls, and reporting recommendations for pT1 disease. In T1 disease, several substaging strategies have been proposed to improve outcome prediction (25, 26, 27) but have been difficult to adopt due in part to the inherent lack of orientation of the specimen. One strategy separates T1 disease into that above the muscularis mucosae (T1a), below the muscularis mucosae but above the venous plexus (T1b), and beyond the venous plexus (T1c) or separates microinvasive (T1m) disease from more deeply invasive disease (T1e) (25, 28). Whereas T1a and T1b corresponded to a 5-year survival rate of 75%, T1c disease was associated with a 5-year survival rate of only 11% (29). A second strategy separates superficial from deep lamina propria invasion into T1a and T1b categories, respectively, with T1b showing higher rates of progression (58% vs. 36%) and death from disease (46% vs. 23%) than T1a (30). Finally, the depth of invasion, a maximum diameter of invasive carcinoma of less than 1 high-power field versus ≥1 high-power fields (irrespective of direction), and a cutoff of 1 mm maximum invasive diameter have all been shown to be associated with prognosis (31). Based on the available data, it is recommended to provide an assessment of the depth and/or extent of subepithelial tissue invasion in T1 cases.
TABLE 7.2 American Joint Committee on Cancer Staging System for Bladder Cancer (2009) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree