Mayo Clinic Referral Population
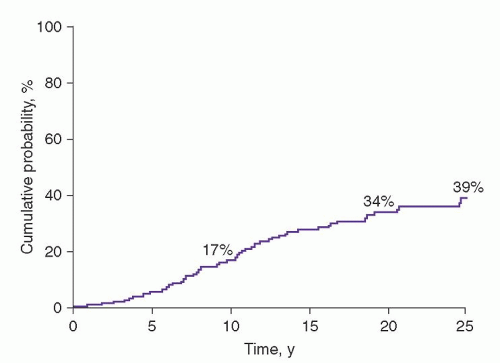
The prognosis of MGUS was first established in a study of 241 patients seen at the Mayo Clinic from 1956 through 1970.
1 The actuarial rate of progression to MM or related disorder at 10 years was 17%; at 20 years, 34%; and at 25 years, 39% (
Fig. 97.4).
72 Of the 64 patients with progression, 44 (69%) had MM.
Southeastern Minnesota Study
The risk of progression has also been estimated in a larger population-based study of 1,384 persons with MGUS who resided in the 11 counties of southeastern Minnesota, the risk of progression of MGUS to MM or related disorder was found to be 1% per year.
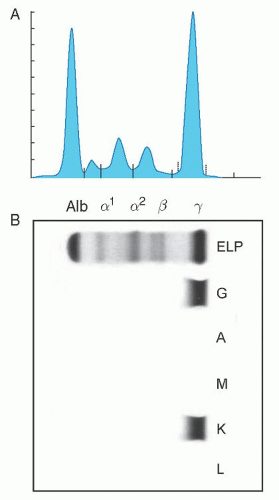
2 The median age at diagnosis of MGUS was 72 years. The M-protein level at diagnosis ranged from unmeasurable to 3.0 g/dl. On the basis of the heavy-chain type of immunoglobulins, 70% of the M proteins were IgG, 12% IgA, and 15% IgM. A biclonal gammopathy was found in 45 patients (3%). The light chain type was
κ in 61% and
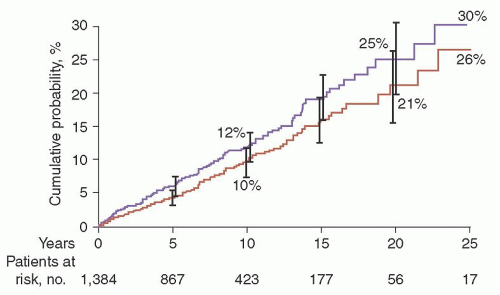
λ in 39%. A reduction of uninvolved (normal or background) immunoglobulins was found in 38% of 840 patients in whom quantitation of immunoglobulins was determined. The 1,384 patients in this study were followed up for a total of 11,009 person-years (median, 15.4 years; range, 0 to 35 years). During follow-up, MM, primary AL, lymphoma with an IgM serum M protein, WM, plasmacytoma, or chronic lymphocytic leukemia developed in 115 patients (8%). The cumulative probability of progression to one of these disorders was 10% at 10 years, 21% at 20 years, and 26% at 25 years (
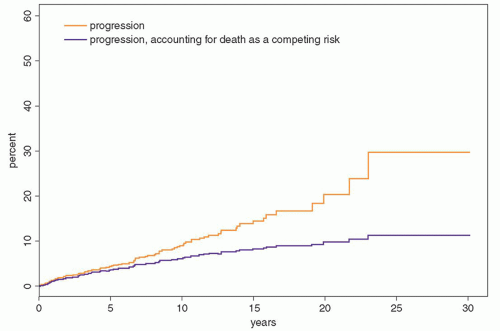
Fig. 97.5). Patients were at risk for progression even after 25 years or more of stable MGUS. Although the risk of progression is 1% per year, it must be emphasized that this does not take into account other competing causes of death in elderly patients. After adjusting for competing causes of death, the true lifetime probability of progression of MGUS for the average patient is only approximately 10% (
Fig. 97.6).
The number of patients with progression to a plasma cell disorder (115 patients) was 7.3 times the number expected on the basis of the incidence rates for those conditions in the general population (
Table 97.4). The risk of MM developing was increased 25-fold; WM, 46-fold; and AL amyloidosis, 8.4-fold. The risk of development of lymphoma was only modestly increased at 2.4, but this risk was underestimated because only lymphomas associated with an IgM protein counted in the observed number, whereas the incidence rates for lymphomas associated with IgG, IgA, and IgM proteins were used to calculate the expected number. The risk of development of chronic lymphocytic leukemia was only slightly increased.
The 75 patients in whom MM developed accounted for 65% of the 115 patients who had progression to a plasma cell disorder. The mode of development of MM among the patients with MGUS was variable. The M-protein level increased within 2 years of the recognition of MGUS in 11 patients, whereas the serum M-protein level was stable for more than 2 years and then increased within 2 years in 19 patients; in 9 others, the M-protein level increased gradually after having been stable for at least 2 years. In 9 patients, the M-protein level increased gradually during
follow-up until the diagnosis of symptomatic MM was made.
In 10 patients, the serum M-protein level remained essentially stable; the diagnosis of MM was unequivocal in these 10 patients because of an increase in bone marrow plasma cells, development of lytic lesions, or occurrence of anemia, renal insufficiency, or an increased level of urine M protein. Seventeen patients had an insufficient number of serum M-protein measurements to determine the pattern of increase. In patients with WM, the M-protein level showed a gradual increase in 3, stable levels were followed by a sudden increase in 2, and data were insufficient in 2.
Spontaneous disappearance of M protein after the diagnosis of MGUS was rare.
2 The M protein disappeared without an apparent cause in 27 patients (2%), and only 6 of these 27 patients (0.4% of all patients) had a discrete spike on the densitometer tracing of the initial electrophoresis (median, 1.2 g/dl); the rest had small M proteins detected on immunofixation only.
Follow-up in Other Series
The risk of progression of MGUS has been estimated in several other studies, and the results mirror those seen in the Southeastern Minnesota study. For example, Baldini et al. noted that 6.8% of 335 patients with MGUS had progression during a median follow-up of 70 months.
73 In the Danish Cancer Registry, 64 new cases of malignancy (5 expected; relative risk, 12.9) were found among 1,229 patients with MGUS.
74 


 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access