- Glycated hemoglobin (HbA1c) levels will increasingly be presented using the International Federation of Clinical Chemistry standard expressed as mmol/mol of unglycated hemoglobin. The equivalent of the current HbA1c levels of 6.5% and 7.5% are 48 mmol/mol and 59 mmol/mol.
- For patients with type 1 diabetes (T1DM), blood glucose control should be monitored with measurement of HbA1c every 2–6 months depending on the level and stability of blood glucose control and change in therapy.
- Patients with T1DM should be encouraged to self-monitor blood glucose with capillary blood glucose meters. With treatment regimens intended to produce intensive glycemic control testing should be frequent (e.g. four or more times a day).
- For patients with type 2 diabetes (T2DM), blood glucose control should be monitored using high precision methods for measurement of HbA1c every 2–6 months depending on the level and stability of blood glucose control and change in therapy.
- For non-insulin-treated patients with T2DM, self-monitoring of blood glucose is unlikely to be cost-effective for well-controlled patients and should therefore only be used when training and support is available and a clear purpose for use identified.
Why monitor?
“The overall goal of diabetes management is to achieve as near normal physiological or ideal values as possible, without detriment to quality of life and, for glucose control in particular, without causing significant hypoglycemia” [1].
Diabetes is a disorder of glucose homeostasis. It currently affects 285 million people worldwide and is expected to affect 435 million by 2039 [2]. For people without diabetes, glucose levels are maintained in the range of 4–6 mmol/L (80–110 mg/dL). When blood glucose levels increase as a result of glycogen conversion or eating carbohydrate-containing food, insulin is released restoring homeostasis through hepatic conversion of glucose to glycogen, and uptake of glucose into muscle and fat cells. Conversely, if blood glucose levels fall too low as a result of exercise or lack of food, glucagon is released causing hepatic conversion of glycogen to glucose.
People with type 1 diabetes mellitus (T1DM) lack the normal homeostatic mechanism to control levels of blood glucose, while people with type 2 diabetes mellitus (T2DM) have an impaired or absent response. In addition to insulin, which is the most important of the regulatory mechanisms, growth hormone, thyroxine and catecholamines are also important counter-regulatory hormones and lead to increases in blood glucose levels. The aim of monitoring glycemic control is to diagnose the nature of any impairment of homeostatic mechanisms, allow patients to understand the nature of their disorder, determine optimum times for initiating therapeutic intervention; and guide the day-to-day adjustment of management regimens.
Glycated hemoglobin (HbAlc) and blood glucose are the two most frequently used measures of glycemia in current practice. Glycated hemoglobin provides information about overall control of glucose levels in the previous 6–8 weeks allowing assessment of the need for therapy and therapeutic response with minimal within-person variation in measurement. Blood glucose concentrations provide information about the day-to-day level of control, variation in control and response to therapeutic intervention. Measurement and interpretation of blood glucose, however, may be difficult because of potentially wide within-person variations in measures other than when fasting. Prospective observational and intervention studies have confirmed that HbA1c levels are related to long-term disease outcomes [3,4]. Both HbA1c and blood glucose therefore, along with a number of other tests, including measurement of urinary glucose for those who do not intend to aim for intensive glucose control, remain an option for helping to identify poor glycemic control and to facilitate the adjustment of therapy to achieve optimal glucose levels.
Tests and their characteristics
Glycated hemoglobin
Quality controlled HbA1c measurement has a central role in the management of diabetes. Its pivotal role derives from its use in reports of the major outcome studies [5,6]; HbAlc levels can be directly related to the risk of development of diabetic complications. Major national guidelines address this area and make recommendations about its use [5,7].
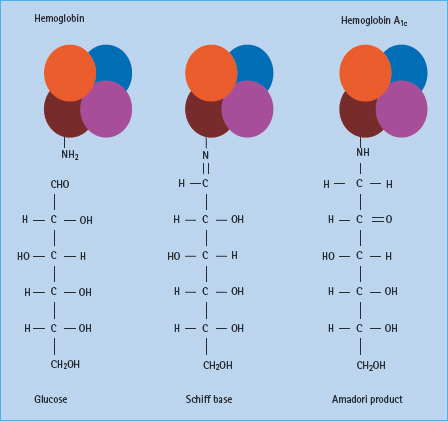
Hemoglobin reacts spontaneously with glucose to form glycated derivatives in a non-enzymatic manner. The process occurs slowly, with the extent of glycation determined by the concentration of glucose in blood. Human hemoglobin A undergoes such glycation to form HbA1c from a reaction between the β-chain of hemoglobin AO and glucose (Figure 25.1). Other compounds result from similar reactions on the α and β chains of hemoglobin and these can be measured as the total glycated hemoglobin.
Until the measurement of different glycated derivatives in the late 1990s, the difficulties of obtaining a common reference standard and the costs of the test made comparisons between laboratories difficult and availability limited. Many of these problems have been overcome, and the most common measured component, HbA1c, is now widely accepted as a standard measurement of glycemic control.
Glycated hemoglobin can be affected by the presence of hemoglobin variants and uremia, but specific assays (e.g. high performance liquid chromatography rather than immunochemistry or affinity chromatography methods) can be used to obtain an accurate result. Vitamin C, hemolytic and iron deficiency anemia can also give abnormal results. Laboratories still differ in whether a result from a heterozygous patient with variant hemoglobin is reported as non-comparable to the Diabetes Control and Complications Trial/UK Prospective Diabetes Study (DCCT/ UKPDS) standard, or whether it is not reported at all. Other conditions in which there is a rapid turnover of erythrocytes (e.g. polycythemia, anemia or blood transfusion) can also give inaccurate results (Table 25.1).
Table 25.1 Conditions that can affect the measurement of glycated hemoglobin.
| Iron deficiency anemia Hemoglobinopathies Polycythemia Blood transfusion Hemolysis (hemolytic anemia) Uremia caused by renal failure High levels of vitamin C |
Glycated hemoglobin serves as a retrospective indicator of the average glucose concentration over the previous 6–8 weeks. Approximately 50% of the variance in HbA1c is determined by the average blood glucose concentration over the previous month, 25% by the concentration over 30–60 days, and the remaining 25% by the concentration from 60 to 120 days.
Alignment of assays for HbA1c
New International Federation of Clinical Chemistry standard
Soon after glycated hemoglobin measurements were adopted into routine use, it became clear that there were significant differences between results from differing laboratories. The differences arose because of the range of assays used and lack of agreement over a common reference standard. With the publication of DCCT [5] in 1993 a number of countries developed national programs to standardize measurement, which included external quality assurance schemes to ensure that measurements between laboratories could be compared [8]. With the development of more advanced assays it became possible to propose a new reference method based on assaying a specific component of HbA1c (the β-N-terminal hexapeptide).
Despite these efforts to standardize HbA1c there are over 30 different methods in use for measurement. Manufacturers provide calibration factors for individual machines, and there is a global network of laboratories that maintain and monitor the relationship between the different standards.
The new reference standard, agreed by the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) was not initially viewed with enthusiasm because it would have lowered the reference range of HbA1c by 1.5–2.0 percentage points, thus leading to confusion among both clinicians and patients and a potential deterioration in population glycemic control. A shift to the new reporting strategies, however, offers the opportunity for a global agreement about the requirements for manufacturers to supply equipment that can be standardized.
In Europe the new standard will be introduced to ensure laboratory and clinical practice is traceable to the IFCC reference method using units expressed as mmol per mol of unglycated hemoglobin. In addition, all results will be presented with both the DCCT aligned and the IFCC standard until at least June 2011. Thus, when HbA1c results (DCCT aligned) are expressed as percent hemoglobin the equation deriving the relationship is:
IFCC-HbA1c (mmol/mol) = [DCCT-HbA1c (%)– 2.15] × 10.929
The conversion of integer HbA1c (%) values to new units has an easy-to-remember numerical relationship known as “Kilpatrick’s kludge” [9]. For example, for an HbA1c of 7% subtract 2 = 5, and then subtract a further 2 = 3. Thus, a HbA1c of 7% becomes 53 mmol/mol. For non-integer values the use of the formula or reference tables is required. The equivalent of the current HbA1c targets of 6.5% and 7.5% are 48 mmol/mol and 59 mmol/mol in the new units (Table 25.2).
For both patients and clinicians, a shift to the new standard will reduce potential confusion between blood glucose levels and HbA1c measurements. With current reference ranges it is relatively easy for confusion between a blood glucose value given in mmol/L to a value of a similar order to the HbA1c (%) level but having different implications for clinical decision-making. Nevertheless, a considerable education initiative is needed to inform both patients and clinicians of the change.
Table 25.2 IFCC aligned values for HbA1c.
| Current DCCT aligned HbA1c (%) | IFCC HbA1c (mmol/mol) |
| 4.0 | 20 |
| 5.0 | 31 |
| 6.0 | 42 |
| 6.5 | 48 |
| 7.0 | 53 |
| 7.5 | 59 |
| 8.0 | 64 |
| 9.0 | 75 |
| 10.0 | 86 |
IFCC-HbA1c (mmol/mol) = [DCCT-HbA1c (%) – 2.15] × 10.929
Estimated average glucose measurements
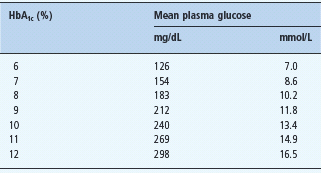
As glycated hemoglobin levels are related to blood glucose concentrations and formulas, the American Diabetes Association (ADA) has proposed an alternative approach with the introduction of the estimated average glucose measurement (eAG).
Glycated hemoglobin provides information about the overall levels of glucose to which tissue is exposed. For a non-insulin-using patient with T2DM with regular fasting plasma glucose (FPG) levels of 6.5 mmol/L, an HbA1c of around 53 mmol/mol (7%) would be expected, but prandial elevation of blood glucose makes an additional contribution to the rate of glycosylation, thus affecting individual results [10].
A large international study has recently established a strong correlation between continuous blood glucose monitoring results and HbA1c (r = 0.92) in 507 Caucasian adults. The authors proposed a formula to derive eAG [11]; and led to the recent ADA position statement recommending the use of the eAG measure [5]. Concerns about the extent to which the relationship between HbA1c and eAG are generalizable, the limited information about applicability to all ethnic groups and the potential for patient confusion about the measurements have led to limited adoption outside the USA.
The extent to which eAG are related to diabetes complications is controversial. There are no long-term studies relating continuous blood glucose measurement to long-term diabetes outcomes. Instead, HbA1c remains both a target of intervention in many trials, as well as a measure of success in improving glycemic control. This approach is based on the demonstrated relationships between HbA1c and diabetes outcomes. There is therefore concern that the introduction of an eAG might lead patients and clinicians to place weight on measurement of blood glucose, while the assay is actually of HbA1c.
Use of HbA1c in the diagnosis of diabetes
Current guidance continues to recommend that the diagnosis of diabetes is based on at least two laboratory measurements of blood glucose ≥7 mmol/L or random samples of ≥11.1 mmol/L. In the event of uncertainty, an oral glucose tolerance test is proposed. HbA1c offers a potentially easier, non-fasting and therefore more acceptable test. Furthermore, there appears to be less intra-individual variation with HbA1c than glucose testing. Previously, there were concerns that despite the variation in results of oral glucose testing within the same person, the alternative of a single HbA1c test would be unsatisfactory because the threshold for screening (usually identified as 53 mmol/mol [7%]) would miss a substantial number of people with diabetes. There is also considerable potential for confusion, because current guidance for people with diabetes is to aim, if possible, for HbA1c levels of 48–59 mmol/mol (6.5–7.5%) [7]. One possible alternative to simplify diagnosis is the combined measurement of fasting blood glucose and HbA1c, making use of an algorithm developed from studies in which the two measurements were compared against the results of an oral glucose tolerance test [12]. There are ongoing discussions about the use of HbA1c of a diagnostic test and, at the time of writing, it has been proposed that an HbA1c of 48 mmol/ mol (6.5%) would be diagnostic of diabetes. This test will not replace the glucose criteria as in many parts of the world HbA1c is not available.
Point of c are HbA1c
Point of care testing for HbA1c is now possible with clinic-based analyzers and may allow timely decisions on therapy changes when needed. A recent study of their use, however, carried out in a clinic setting, does not yet provide sufficient information about the benefits to justify their widespread deployment [13,14].
Measurement of fructosamine
Albumin is the main component of plasma proteins. Albumin contains free amino groups which can react non-enzymatically with glucose to form fructosamine. Measurement of fructos-amine reflects glucose levels over the previous 1–3 weeks. Fructosamine measurement is not appropriate for routine use because the assay is markedly affected by excessive turnover or excretion of albumin in, for example, renal disease. In addition, fructosamine assays are not standardized in the same way as HbA1c assays. Fructosamine testing remains useful in a number of circumstances, for example in pregnancy where glucose requirements change rapidly, or in the preconception period where motivation to improve control is high and the changes can be evaluated at shorter intervals. With the lower cost and increased standardization of HbA1c, however, it is possible that more frequent measurements of HbA1c may be an alternative strategy, particularly if evaluating potentially large improvements in the blood glucose over the preceding 4–6 weeks contributing to a clinically important change from a previous HbA1c result [15].
Blood glucose
Blood glucose (or the level of glucose to which the body’s organs are exposed) is expressed as the plasma glucose concentration. Blood glucose was traditionally given as whole blood values, but plasma glucose levels are now measured and reported by most laboratories. Blood samples for analysis need to be taken under controlled conditions to allow accurate measurement of glucose levels. Red blood cells may continue to metabolize glucose after collection, continuing glycolysis, and thus leading to reductions in glucose levels. This should be avoided by rapid centrifugation, collection onto ice and storage in a refrigerator. If blood is to be transported at room temperature it should be collected into fluoride-containing tubes which inhibit further glucose metabolism. If a patient has an intravenous line in situ, blood should be drawn from the arm opposite to the one with the line to prevent contamination of the sample from any infusion.
Blood glucose measurement is a term that is frequently used without precise definition. Measurement of glucose levels is usually carried out either on capillary or venous samples of blood. Most laboratories measure the level of glucose in plasma, which again differs from measurements made on whole blood. Table 25.3 shows the equivalent measurements from the different sites and samples taken.
Blood glucose levels are expressed in SI (Systeme International) units as millimoles/liter (mmol/L). The traditional unit for measuring blood glucose is milligrams/decilitre (mg/dL), although use of these units is now largely confined to the USA. To convert mmol/L glucose to mg/dL, multiply by 18 (Table 25.4).
Table 25.3 Values for diagnosis of diabetes mellitus.
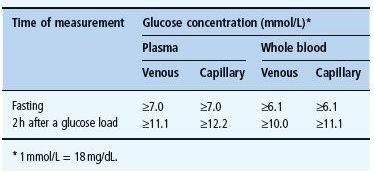
Table 25.4 Correlation of HbA1c with average glucose.
Sampling and preparation of the sample affect measurement. Whole blood glucose is also affected by the concentration of protein (mainly hemoglobin) in the sample. Whole blood concentrations are therefore 12–15% lower than plasma concentrations. Venous blood glucose levels are normally similar to arterial and capillary levels when fasting. The arterial and capillary levels most closely reflect the glucose concentrations at the organ level. After meals, venous blood will have lower glucose concentrations than arterial blood and can be as much as 10% lower.
Analytical techniques and quality assurance
A range of analytical techniques are used for the laboratory measurement of blood glucose levels. Chemical oxidation/reduction methods have a low cost for reagents and, although less specific, are still valid. Enzymatic analysis of glucose is more specific, although more expensive. The enzymatic reference method for glucose is the hexokinase/G6PDH method. The glucose oxidase methods are comparable, although the presence of reducing substances may cause error. Glucose oxidase methods are frequently used because of their convenience and lower cost. Measurements are accurate and precise with measurement coefficient of variance of around 2%. Self-measurement of blood glucose is possible using capillary blood glucose meters with test strip systems. Specific issues associated with their use are considered in the next section.
Capillary blood glucose meters use test strips that release gluconic acid and hydrogen peroxide from a blood sample. The reaction is quantified by one of a range of methods to measure blood glucose. Most of the currently marketed handheld capillary blood glucose meters give results as an equivalent to venous plasma glucose but this is not always the case. The same type of handheld meter may be calibrated to report whole blood glucose in one country and plasma values in another. Until this issue is resolved, the calibration of a meter should be checked and the thresholds for action set accordingly.
In a hospital or “site-of-care” setting, capillary blood glucose measurement can be used to replace venepuncture, with greater comfort and more rapidly available results. Standards have been laid down to ensure that bedside glucose determinations can be made accurately and include the need for well-defined policies which include adequate training, quality control procedures and regular maintenance of equipment [16]. Blood glucose meters may require entry of a number or insertion of a coding chip to ensure calibration to the batch of testing strips used.
Accuracy of blood glucose meters
Although laboratory methods of blood glucose measurement are accurate, the convenience and rapidity of capillary blood glucose meters means that, despite their higher coefficient of variance compared to laboratory testing and the possibility of user error, they are in wide use. The majority conform to international standards (www.iso.org), with 95% of readings within 0.83 mmol/L for readings <4 mmol/L and within 20% for higher glucose readings. The newer meters have minimized the possibility of user error by requirement for smaller volumes of blood for measurement and automated calibration methods. Operator error, however, remains a significant source of error, including failure to calibrate meters (some newer meters do not require external calibration), poor handwashing technique and dirty meters [17].
Measurement error can be minimized by careful training and consistent technique in making measurements, incorporating an allowance for the possibility of error in the reading when calculating insulin dose, and undertaking regular testing to identify results that do not fit the usual pattern, with retesting as necessary.
Despite their imprecision, blood glucose meters remain helpful at higher blood glucose values, where, for example, it is of less importance to distinguish a plasma glucose of 11 mmol/L from one of 14 mmol/L. In such circumstances, the aim of management is to achieve a substantial reduction in plasma glucose. At lower plasma glucose levels, however, the consequences of an imprecision of 15% are much greater. For many well-controlled patients aiming to keep their glucose levels in the range 4–6 mmol/L, the majority of readings below 4 mmol/L will result from the variance of the measurement rather than reflecting a true “low” value.
Measurement of urinary glucose
Measurement of glucose in urine has limited arguments in favor of its use for routinely monitoring diabetes. It is rapid, inexpensive, non-invasive and can provide a quantitative result, however, it does not reflect the changing levels of hyperglycemia with any accuracy and so interpretation may be difficult if not impossible. In addition, the renal threshold varies between individuals and varies during pregnancy and with aging. In any case, glucose is not excreted renally at levels where blood glucose is significantly elevated above that which should be targeted to minimize diabetic complications.
Urine fraction should be analyzed immediately, preserved at pH <5 to inhibit bacterial metabolism or stored at 4 °C. The enzyme used is glucose oxidase/peroxidase which may lead to false-positive results with hydrogen peroxide and false-negative test results with the presence of ascorbic acid.
Urine testing should no longer be used in most health care settings because of the availability of alternative and more accurate tests. For the moment it may have a role in resource-poor settings where identification and treatment of individuals with poorly controlled diabetes is the highest priority.
Monitoring in clinical practice
Glycated hemoglobin measurement is recommended to assess the maintenance of glycemic control and should be measured with high precision methods. Results should be reported in IFCC units (mmol/mol) or eAG (mmol/L or mg/dL) alongside the DCCT aligned (%) units. In general, HbA1c measurements should be performed at least twice a year in patients meeting treatment targets and with stable glycemic control. An interval of 3 months between tests is usually recommended following changes in therapy or when levels are unstable, although a test after 2 months may provide some additional information [18].
Figure 25.2 Correct technique for self-monitoring of blood glucose improves accuracy of testing.
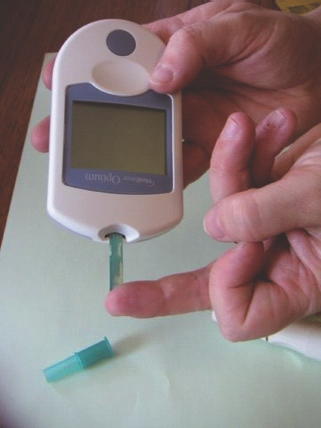
Self-monitoring of blood glucose (SMBG) is a standard of care for patients with T1DM and necessary for insulin-treated patients with T2DM, although optimal use in this group is not established. SMBG may also be helpful for some non-insulin-treated patients with T2DM, although it is unlikely to offer benefit to well-controlled patients. SMBG requires a high level of motivation and if people using SMBG are not aware of how to interpret the results and what actions they should take, it is unlikely to be a clinically effective or cost-effective procedure. People with diabetes need to be taught how to perform the test accurately and how to use the data to adjust therapy in relation to food intake and physical activity. Correct technique involves obtaining the blood sample from the side of the finger pulp, wiping and using the second drop of hanging blood, using the meter correctly and disposing of the lancet (Figure 25.2). Alternative sites for sampling include the base of the thumb, forearm and thigh. Pre-meal readings will be the same between sites, but at times of rapid glucose change (in the post-prandial period or during hypoglycemia) forearm and thigh results will be different from the fingertip results.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree