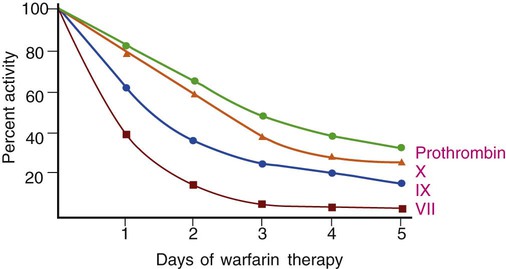
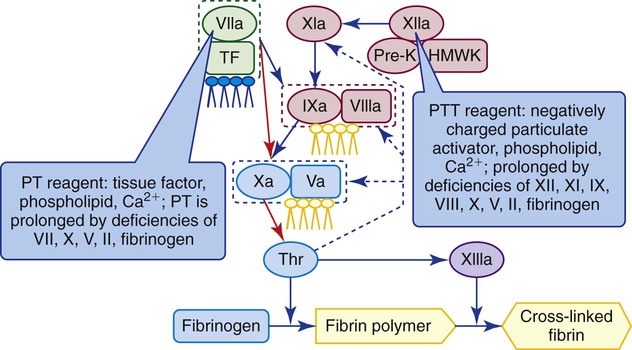
After completion of this chapter, the reader will be able to: 1. Describe the purpose of antithrombotic administration and distinguish between anticoagulants and antiplatelet therapy. 2. Describe the indications for, dosage of, and management of warfarin therapy, including how to treat warfarin overdose. 3. Monitor warfarin therapy using the prothrombin time and international normalized ratio, and compare these tests with the chromogenic X assay. 4. Perform and interpret prothrombin times using point-of-care instruments. 5. Describe the indications for, dosage of, and management of unfractionated heparin therapy, including how to establish the unfractionated heparin therapeutic partial thromboplastin time range. 6. Describe the indications, dosage, and management of low-molecular-weight heparin therapy and fondaparinux therapy. 7. Perform and interpret the results of the partial thromboplastin time and activated clotting time assays for the monitoring of unfractionated heparin therapy, and compare these tests with the chromogenic anti-Xa heparin assay. 8. Perform and interpret the results of the chromogenic anti-Xa assay for the monitoring of unfractionated heparin therapy, low-molecular-weight heparin therapy, and fondaparinux therapy. 9. Select appropriate laboratory tests for the monitoring of antithrombotic therapy, recognize appropriate testing frequency, follow appropriate timing for test administration, and interpret test results as indicating adequate, inadequate, or excessive drug dosage. 10. Describe how rivaroxaban exerts its direct anti-Xa effect; discuss methods for monitoring rivaroxaban therapy. 11. Monitor therapy with direct thrombin inhibitors, including dabigatran, using the partial thromboplastin time and the ecarin clotting time. 12. Describe the therapeutic function of the intravenous platelet glycoprotein IIb/IIIa inhibitors and describe how their effects are monitored. 13. Describe the therapeutic function of the oral platelet inhibitors aspirin, clopidogrel, ticlopidine, and prasugrel and describe how their effects are monitored. Venous or arterial thrombotic disease is managed by antithrombotic therapy, which includes anticoagulant and antiplatelet therapy. Venous thromboembolic disease (venous thromboembolism, or VTE) includes superficial and deep vein thrombosis (DVT) and pulmonary embolism (PE) and is treated using the anticoagulants warfarin (Coumadin), standard unfractionated heparin (UFH), low-molecular-weight heparin (LMWH, enoxaparin), and synthetic pentasaccharide (fondaparinux). The direct thrombin inhibitors (DTIs) argatroban, lepirudin, and bivalirudin are additional anticoagulants substituted for heparin in patients who have developed heparin-induced thrombocytopenia (HIT) subsequent to UFH therapy (see Chapter 42). All of these anticoagulant drugs are administered intravenously except warfarin, which is an oral anticoagulant. Unfractionated heparin therapy began in the late 1930s, and warfarin therapy was first used in 1952. As of July 2010, warfarin remained the only oral anticoagulant, although at least two new oral anticoagulants, rivaroxaban and dabigatran, may be cleared by the U.S. Food and Drug Administration (FDA) in 2011 or 2012. Many lives have been saved through judicious use of antithrombotic therapy, and countless more healthy individuals have been spared thrombotic disease through long-term antithrombotic prophylaxis. However, antithrombotics are dangerous because their effective dosage ranges are narrow.1 Overdose is critical and leads to emergency department visits for uncontrolled bleeding; inadequate dosages lead to secondary (repeat), often fatal, thrombotic events. Dosages and half-lives differ among the antithrombotics and thrombolytics because of variations in formulation and metabolism.2,3 Because of these risks, laboratory monitoring of antithrombotic therapy, the earliest form of therapeutic drug monitoring, is essential. Coagulation laboratory scientists perform countless prothrombin time (PT) assays, partial thromboplastin time (PTT, activated partial thromboplastin time [APTT]) assays, and chromogenic anti–factor Xa heparin assays to monitor anticoagulant therapy, and this accounts for most of their workload. Antiplatelet therapy monitoring generates a smaller, but rapidly growing workload. Although antithrombotic therapy monitoring may seem routine, vigilance is essential to provide consistently valid results in a dangerous therapeutic world.4 As detailed in Chapter 40, coagulation factors II (prothrombin), VII, IX, and X depend on vitamin K for normal production, as do coagulation control proteins C, S, and Z. Vitamin K is responsible for γ-carboxylation of a series of 12 to 18 N-terminus glutamic acids, a posttranslational modification that enables these coagulation factors and control proteins to bind ionic calcium (Ca2+) and cell membrane phospholipids, especially phosphatidylserine (see Figure 40-8). Vitamin K is concentrated in green leafy vegetables and is produced by gut flora; its absence results in the production of nonfunctional des-γ carboxyl, forms of factors II, VII, IX, and X and proteins C, S, and Z. Warfarin sodium (4-hydroxycoumarin, Coumadin) is a member of the coumarin drug family and is the formulation of coumarin most often used in North America.5 Another coumarin is dicumarol (3,3′-methylenebis-[4-hydroxycoumarin]), the original anticoagulant extracted from moldy sweet clover by Link in 1940.6 Warfarin is a vitamin K antagonist that suppresses γ-carboxylation of glutamic acid by slowing the activity of the enzyme vitamin K epoxide reductase (see Figure 40-8). During warfarin therapy, the activities of factors II, VII, IX, and X and proteins C, S, and Z become reduced as the nonfunctional des-carboxyl proteins are produced. These are called proteins induced by vitamin K antagonists (PIVKAs); they bind few calcium ions, do not assemble on phospholipid surfaces with their substrates, and therefore do not participate in coagulation. Despite several developmental efforts, in 2010 coumarins remained the only oral anticoagulants in the United States, so warfarin therapy is often called oral anticoagulant therapy. Two new oral anticoagulant drugs, rivaroxaban and dabigatran, became available in 2009 outside the United States and may be cleared by the FDA in 2011 or 2012. The standard warfarin regimen begins with a 5-mg daily oral dose. The starting dosage for people older than 70 years and people who are debilitated, malnourished, or have congestive heart failure is 2 mg/day. For people simultaneously taking drugs that are known to raise warfarin sensitivity, the starting dosage is 2 mg/day, and 2 mg/day is also the dosage used for those with inherited warfarin sensitivity. There is no loading dose, and subsequent dosing is based on patient response as measured by the PT (see the next section). The activity of each of the vitamin K–dependent coagulation factors begins to decline immediately but at different rates (Figure 46-1) for each factor, and it takes about 5 days for all the factors to reach therapeutic levels. Table 46-1 lists the plasma half-life, plasma concentration, and minimum effective plasma percentage of normal factor activity for the coagulation factors. TABLE 46-1 Control protein activities also become reduced, especially the activity of protein C, which has a 6-hour half-life, so for the first 2 or 3 days of warfarin therapy the patient actually risks a thrombotic event. For this reason, warfarin therapy is “covered” by UFH, LMWH, or pentasaccharide therapy for at least 5 days. Failure to provide anticoagulant therapy during this period may result in warfarin skin necrosis, a severe reaction requiring débridement of dead tissue.7 The PT effectively monitors warfarin therapy because it is sensitive to reductions of factors II, VII, and X (Figure 46-2). The PT reagent consists of tissue factor, phospholipid, and ionic calcium, so it triggers the coagulation pathway at the level of factor VII. Owing to the 6-hour half-life of factor VII, the PT begins to prolong within 6 to 8 hours; however, anticoagulation becomes therapeutic only when the activities of factors II and X decrease to less than 50% of normal, in approximately 5 days. The medical laboratory scientist reports PT results to the nearest tenth of a second and provides the PT reference interval for comparison. In view of the inherent variations among thromboplastin reagents, and to accomplish interlaboratory normalization, all laboratories report the international normalized ratio (INR) for patients with a stable response to anticoagulant therapy using the following formula8: where PTpatient is the PT of the patient in seconds, PTnormal is the geometric mean of the PT reference interval in seconds, and ISI is the international sensitivity index applied as an exponent in the formula. Thromboplastin producers generate the ISI by performing an orthogonal regression analysis comparing the results of their PT reagents for 50 or more anticoagulated specimens and 10 or more normal specimens with the published results of the international reference thromboplastin (World Health Organization human brain thromboplastin).9 Most manufacturers provide ISIs for a variety of coagulation instruments, because each may respond differently to their thromboplastins; for instance, some coagulometers rely on photometric changes, whereas others use an electromechanical system (see Chapter 47). Most thromboplastin reagents have ISIs near 1, which is the ISI of the World Health Organization’s thromboplastin. Automated coagulation timers request the reagent ISI from the operator or obtain it directly from a reagent vial bar code and compute the INR for each assay result. Although INRs are meant to be computed only for patients taking anticoagulants in whom the response to therapy has stabilized, they typically are reported for all patients, even those who are not taking warfarin. During the first 5 days of warfarin therapy, the astute physician and medical laboratory scientist ignore the INR as unreliable and interpret the PT results in seconds, comparing it with the reference interval. Two polymorphisms generate variations in enzymes of the cytochrome P-450 pathway. These are CYP2C9*2 and CYP2C9*3, which reduce pathway activity and slow the metabolic breakdown of warfarin. Likewise, there is a polymorphism that affects the key enzyme of vitamin K metabolism, vitamin K epoxide reductase. This polymorphism, named VKORC1, slows vitamin K reduction, which makes the patient more sensitive to warfarin. In patients possessing one, two, or all three of these polymorphisms, warfarin therapy should begin at 2 mg/day and should be adjusted and monitored daily until the INR remains consistently in the therapeutic range. The standard 5 mg/day regimen risks hemorrhage in these patients. In 2007, the FDA required that drug manufacturers add a statement on all vials of warfarin recommending that physicians screen patients for dosage-affecting polymorphisms. Although the FDA recommendation does not carry the weight of a black box warning, numerous molecular diagnostics manufacturers have developed short turn-around assays for these three polymorphisms. Screening for these polymorphisms is the first and most public example of pharmacogenomic laboratory testing, although as of 2010 it had not been universally endorsed.10 Conversely, warfarin receptor insufficiency may render the patient resistant to warfarin therapy. Some patients require dosages of 20 mg/day or higher to achieve a therapeutic INR. The search is on for polymorphisms responsible for such “warfarin resistance,“ and by 2012 or 2013, at least one such assay may become available from reference laboratories.11 The intravenously administered DTIs argatroban, lepirudin, and bivalirudin, which are used in place of heparin as a life-saving measure for patients with HIT, prolong the PT, although the PTT is the assay typically employed to monitor DTI therapy. In subsequent switching from DTI to warfarin therapy, the clinician is aware that the combination of a DTI and warfarin nearly doubles the PT for the duration of action of the DTI, which is 3 or 4 days. Argatroban exerts the greatest effect on the PT, followed by bivalirudin and lepirudin.12 The chromogenic X assay is an effective means for monitoring warfarin dosage during the crossover period. Table 46-2 provides recommendations for the reversal of a warfarin overdose based on INR and clinical evidence of bleeding. The concerns surrounding warfarin therapy will recede in importance as new oral anticoagulants are cleared for distribution in the United States (see the sections on rivaroxaban and dabigatran). TABLE 46-2 • Cascade POC, Actalyke XL and Actalyke Mini II (Helena Point of Care, Beaumont, Tex.) • CoaguChek XS PT test system and CoaguChek XS Plus PT test system (Roche Diagnostics, Indianapolis, Ind.) • Gem PCL Plus (Instrumentation Laboratory, Bedford, Mass.) • HMS Plus, ACT Plus (bench top, Medtronic Cardiac Surgery, Minneapolis, Minn.) • INRatio PT INR monitor, INRatio2 PT INR monitor (HemoSense/Inverness Medical, San Diego, Calif.) • i-STAT 1 (Abbott Point of Care, Inc., Princeton, N.J.) • ProTime Microagulation System—New ProTime; Hemochron Signature Elite; Hemochron Signature Plus; Hemochron Response (International Technidyne Corporation, Edison, N.J.) Most of these instruments are portable and are hand-held.13
Monitoring Antithrombotic Therapies
Warfarin Therapy and the Prothrombin Time
Warfarin: Vitamin K Antagonist
Warfarin Prophylaxis and Therapy
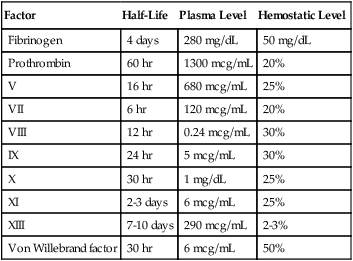
Factor
Half-Life
Plasma Level
Hemostatic Level
Fibrinogen
4 days
280 mg/dL
50 mg/dL
Prothrombin
60 hr
1300 mcg/mL
20%
V
16 hr
680 mcg/mL
25%
VII
6 hr
120 mcg/mL
20%
VIII
12 hr
0.24 mcg/mL
30%
IX
24 hr
5 mcg/mL
30%
X
30 hr
1 mg/dL
25%
XI
2-3 days
6 mcg/mL
25%
XIII
7-10 days
290 mcg/mL
2-3%
Von Willebrand factor
30 hr
6 mcg/mL
50%
Monitoring Warfarin Therapy Using the Prothrombin Time Assay
Reporting Prothrombin Time Results and the International Normalized Ratio

Effect of Polymorphisms on Warfarin Therapy
Effect of Direct Thrombin Inhibitors on the Prothrombin Time
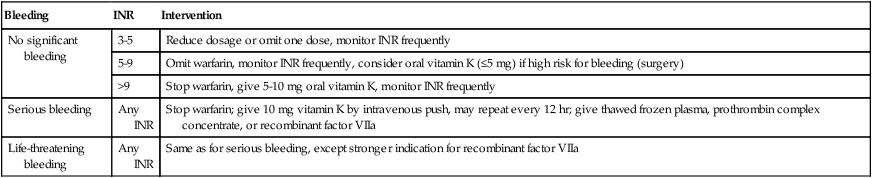
Reversal of Warfarin Overdose
Bleeding
INR
Intervention
No significant bleeding
3-5
Reduce dosage or omit one dose, monitor INR frequently
5-9
Omit warfarin, monitor INR frequently, consider oral vitamin K (≤5 mg) if high risk for bleeding (surgery)
>9
Stop warfarin, give 5-10 mg oral vitamin K, monitor INR frequently
Serious bleeding
Any INR
Stop warfarin; give 10 mg vitamin K by intravenous push, may repeat every 12 hr; give thawed frozen plasma, prothrombin complex concentrate, or recombinant factor VIIa
Life-threatening bleeding
Any INR
Same as for serious bleeding, except stronger indication for recombinant factor VIIa
Prothrombin Time Point-of-Care Testing
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Monitoring Antithrombotic Therapies